#022: A Map of Mitochondria Longevity Companies (PART 2)
Stealth BioTherapeutics, Retrotope, and more!
*This is PART 2 of Newsletter #022: A Map of Mitochondria Longevity Companies (PART 1)
Peroxidation / Oxidation
Stealth BioTherapeutics (NASDAQ:MITO)
Market cap: $94 M
Enterprise Value: $54 M
Cash: $40 M
Founded: 2006
Modality: peptide, injection
Founders: Hazel Szeto
Partnership: Alexion (NASDAQ:ALXN)
Notable Investors:
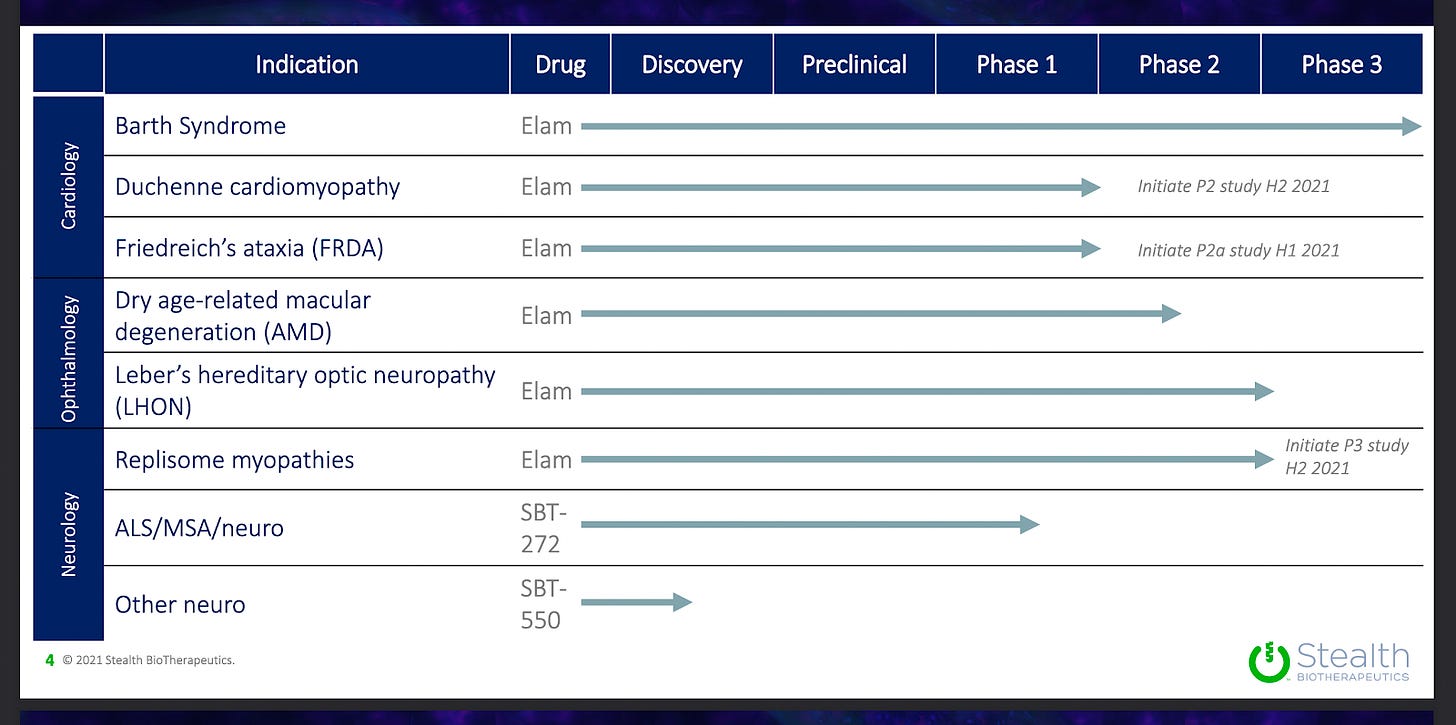
Pipeline:
Elamipretide // Barth Syndrome (rare mitochondrial disease) // Phase 2/3 (active)
Elamipretide // Geographic Atrophy (Dry AMD) // Phase 2 (recruiting)
Elamipretide // Duchenne cardiomyopathy // Phase 2 (planned 2021)
Elamipretide // Freidreich’s Ataxia // Phase 2 (planned 2021)
Elamipretide // Leber Hereditary Optic Neuropathy (LHON) // Phase 2 complete
2nd generation peptides & mimetics // Neurodegenerative diseases // Preclinical
General Notes
Stealth BioTherapeutics is a clinical-stage company developing peptides and peptide mimetics to treat diseases linked to mitochondrial dysfunction.
Their main asset is a four-amino acid peptide called elamipretide. Elamipretide is believed to permeate the outer mitochondrial membrane where it helps reduce the production of Reactive Oxygen Species and stabilize cardiolipin, a phospholipid foundin the inner mitochondrial membrane.
Stealth had a high-profile failure of a Phase 3 trial for elamipretide to treat Primary Mitochondrial Myopathy in December 2019. The stock fell ~66% on the news and down as much as 90% in subsequent months.
Stealth’s lead clinical program is targeting Barth Syndrome, a rare mitochondrial disease. Some early Phase 2 data has shown elamipretide improves 6-minute walking test, cardiac function biomarkers, and Barth Syndrome Scale scores.
Stealth is also developing 2nd generation peptides or peptide mimetics (SBT-272, SBT-550).
Origins & Funding
Hazel Szeto accidentally discovered peptides that act on mitochondria as a researcher at Weill Cornell Medical College studying opioid receptors.
Stealth was eventually spawned out of Morningside Ventures, a venture capital firm founded by a wealthy Hong Kong family. Rennie McCarthy, a lawyer, and Principal at Morningside would eventually be placed as CEO of Stealth.
Morningside is the majority investor in the company and has also injected cash into the company post-IPO.
Pipeline Details
Stealth has two active clinical trials with elamipretide. Most of their planned clinical trials involve elamipretide, with a couple of newer 2nd generation peptides/mimetics being tested for neurological indications
Elamipretide (also known as MTP-131, SS-31, Bendavia) is a four-amino acid peptide. ~0.68 kDa weight. Stealth believes it can permeate the mitochondrial membrane, reduce the levels of Reactive Oxygen Species, and stabilize cardiolipin -- a phospholipid found in the inner mitochondrial membrane. Elampipretide is believed to have many beneficial effects for impaired mitochondria (Review by Szeto et al. 2018):
Elamipretide increases exercise capacity and improves age-related mitochondrial energetic deficits within one hour of administration in mice (Siegel et al. 2013)
Elamipretide has also been shown to reverse cardiac aging in old mice (reduce diastolic dysfunction, reduce proton leak and ROS in mitochondria). (Chiao et al. 2020)
Cardiolipin, which Stealth believes is stabilized by elamipretide, is a mitochondrial membrane phospholipid that has many functions including (Paradies 2019):
Regulating membrane structure
Triggering apoptosis
Mitochondria quality control
Mitochondrial biogenesis, metabolism, and energy production.
Cardiolipin is also very sensitive to oxidative degradation from ROS (“peroxidation”).
Elamipretide // Barth Syndrome // Phase 2/3 (Orphan drug designation)
Barth syndrome is a rare mitochondrial disease characterized by an enlarged and weakened heart and general weakness. It affects roughly 1 in 300,000 people in the United States. There are no FDA-approved treatments.
Barth syndrome is caused by a mutation in the nuclear gene that encodes Tafazzin (TAZ), an enzyme that modulates the remodeling of cardiolipin.
Stealth’s early Phase 2 data suggests elamipretide improves cardiovascular function (+~100m in a 6-minute walking test) at 36 weeks in patients with Barth Syndrome. Adverse events related to treatment were mostly related to the injection procedure or were mild (Thompson et al. 2020).
Elamipretide // Geographic Atrophy (in Dry AMD) // Phase 2 (Orphan drug designation)
Dry age-related macular degeneration is the leading cause of blindness in adults in the US. It is characterized by the degeneration of retinal pigment epithelial cells (RPE) and photoreceptors. The cause is not known but could be linked to mitochondrial dysfunction leading to impaired homeostasis and waste removal of RPE cells (Brown et al. 2018).
There are ~10 million patients with Dry AMD or Wet AMD in the US. Dry AMD is estimated to be a $10 billion dollar market. There are no FDA approved treatments for Dry AMD -- but many competitors are developing treatments (Lineage Cell Therapeutics, GenSight Biologics included)
In Stealth’s ReCLAIM-1 open-label Phase 1 study, 21 patients with Dry AMD (with non-central geographic atrophy) were given 40 mg of subcutaneous elamipretide daily for 24 weeks. The study found modest improvements in BCVA/LLVA (~3 - 5 letters) and normal/low light reading acuity (~1 line gain). Elamipretide was safe with no serious adverse events. (Allingham et al. 2019).
Stealth is currently conducting a Phase 2 Dry AMD study (ReCLAIM-2) and has just finished enrolling all their patients. The expected completion date is March 2022.
Elamipretide // Leber Hereditary Optic Neuropathy (LHON) // Phase 2 completed
LHON is a rare mitochondrial DNA mutation disease that involves atrophy of the retinal ganglion layer, which leads to blindness. It occurs in 1 in 50,000 people. There are no FDA-approved treatments specifically for LHON.
It is hypothesized that the increase in ROS production of the dysfunctional mitochondria causes retinal ganglion cell death through apoptosis. Stealth believes that elamipretide can ameliorate the increased ROS and protect the cardiolipin in the inner mitochondrial membrane from peroxidation.
In a Phase 2 double-blind placebo trial (ReSIGHT), 12 patients with LHON were treated with a topical ophthalmic solution of elamipretide for 52 weeks. No difference in Best Corrected Visual Acuity (BCVA) was reported.
Elamipretide // Duchenne cardiomyopathy // Phase 2 (planned 2021)
Duchenne Muscular Dystrophy (DMD) is a rare X-linked genetic disease that causes muscle weakness including weakness in the heart muscles.
The average life expectancy for patients with DMD is 26.
Elamipretide // Freidreich’s Ataxia // Phase 2 (planned 2021)
Friedreich’s Ataxia is a rare genetic disease that causes neurodegeneration. It is caused by a mutation in the FXN gene that leads to the systemic reduction of the protein frataxin. Frataxin is found in the mitochondria and is associated with the formation of iron-sulfur clusters in the electron transport chain.
Prevalence of ~ 1 in 40,000. No FDA-approved treatments.
It is believed that the accumulation of ROS plays a role in Friedreich’s Ataxia (Lupoli et al. 2017).
Elamipretide or 2nd gen peptide mimetics // Neurological disease (ALS, MSA, etc) // Phase 1
Testing elamipretide to treat POLG / Replisome disorders (mtDNA replication disorders) in a subgroup of their failed Primary Mitochondrial Myopathy
Stealth is planning a Phase 1 trial of healthy volunteers for a new 2nd gen peptide mimetic (SBT-272).
Preclinical and discovery phase for Charcot-Marie-Tooth disease (inherited, nerve damage to arms and legs) and Leigh’s Syndrome (psychomotor regression).
Outlook for Stealth BioTherapeutics
Stealth’s Phase 2/3 trial for Barth Syndrome expected to be completed in June 2021. The company has already had a “pre-NDA” meeting with the FDA.
Dry AMD Phase 2 trial with elamipretide has completed patient enrollment. Completion expected in mid-2022.
Stealth will bring developing and testing next-generation peptide or peptide mimetics to treat neurological diseases including ALS and possibly Alzheimer’s and Parkinson’s.
My Thoughts
Stealth BioTherapeutics is basically a “pipeline in a pill” company. The power of having a therapy that targets some aspect of mitochondrial damage means potential applications to a number of diverse diseases. Unfortunately, Stealth already had one major clinical trial failure with elamipretide (Primary Mitochondrial Myopathy). Now they are feeling the downside (~-80%) of having a “pipeline in a pill”. It is true that they have a couple of non-elamipretide molecules in early development, but ultimately they have ~six current or planned elamipretide clinical trials.
Retroptope
Funding Stage: Series D
Total Funding: $82M
Founded: 2006
Location: Los Altos Hills, California, USA
Modality: small molecule, polyunsaturated fatty acid modified with deuterium
Founders/Team: Charles Cantor, Mikhail Shchepinov, Robert J. Molinari
Notable Investors: Morningside Venture, Mehta Family Ventures, SDL Ventures, Timur Artemev
Pipeline:
Links:
General Notes
Retroptope is a clinical-stage company developing RT001, a deuterated polyunsaturated fatty acid to treat Friedreich’s Ataxia (in Phase 3 trials), Infantile Neuroaxonal Dystrophy (INAD), Progressive Supranuclear Palsy (PSP), Dry age-related macular degeneration (dry AMD), and other diseases associated with mitochondrial dysfunction and/or oxidative damage caused by lipid peroxidation (including neuronal or retinal disease).
RT001 is linoleic acid (a polyunsaturated fatty acid) with two deuterium hydrogen isotopes swapped in for hydrogen at critical sites to slow down lipid peroxidation chain reactions.
Origins and Funding
Founded in 2006 by Charles Cantor (Director of Center for Advanced Biotechnology at Boston University), Mikhail Shchepinov (formerly biochemistry researcher at Oxford University), and Robert J. Molinari.
Employs a “virtual model” for research by collaborating with many universities and research organizations to conduct studies.
Has raised $85M in venture funding from investors including Morningside Ventures, SDL Ventures, among others.
Pipeline Details
Deuterated polyunsaturated fatty acids (D-PUFAs): How do they work?
In short, RT001 (and other D-PUFAs) works by slowing down lipid peroxidation. Lipid peroxidation is believed to have detrimental effects on lipid membranes in mitochondria and neuronal/retinal cell membranes in particular.
Lipids in the inner mitochondrial membrane are prone to peroxidation (oxidation of lipids usually initiated by ROS). Peroxidation happens in a chain reaction where a fatty acid is oxidized into a radical → reacts with a water molecule to form a peroxyl-fatty acid radical → which then reacts with other fatty acids, forming lipid radicals and lipid peroxides in a continuing chain reaction.
The peroxidation chain reaction only ends when the fatty acid radical reacts with another radical or is neutralized by an antioxidant (like vitamin C, etc). The faster the peroxidation is terminated, the better. Conversely, the slower the peroxidation rate, the more easily it can be terminated.
Polyunsaturated fatty acids have two or more carbon-hydrogen double bonds.
In RT001 and other polyunsaturated fatty acids, the carbon in between the two double bonds is the site where the oxidation chain reaction of a lipid begins (“methylene bridge”). And in RT001, the two critical single carbon-hydrogen bonds at that carbon site are swapped for carbon-deuterium bonds.
Deuterium is an isotope of hydrogen (a normal hydrogen atom + one neutron). Swapping it with hydrogen tends to significantly slow down reactions due to something called the kinetic isotope effect. It arises from quantum mechanics because heavier particles have lower vibrational energy states, which leads to larger transition energies.
RT001, taken up by lipid membranes of the mitochondria (and other lipids in cells), slows down the peroxidation chain reaction, thus potentially ameliorating mitochondrial dysfunction. It is believed that only 15 - 20% of PUFAs in the membrane need to be deuterated to have an “anti-catalytic” effect (Hill et al. 2012).
RT001 (deuterated poly-unsaturated fatty acid) // Friedreich’s Ataxia // Phase 3 (orphan drug)
Friedreich’s Ataxia is a rare genetic disease characterized by progressive degeneration of the nervous system and impaired movement. Life expectancy is ~ 40 years.
Friedreich’s Ataxia (FRDA) affects roughly 1 in 40,000 people. There are no FDA-approved treatments.
Friedreich’s Ataxia is caused by reduced levels of expression of the FXN gene (nuclear DNA) that encodes for the protein frataxin found in the mitochondria. Frataxin is associated with sulfur-iron clusters that play a role in the electron transport chain.
Phase 1/2 clinical trials of RT001 (9g per day for 28 days) for (FRDA) showed statistically significant improvement in patient peak power, oxygen consumption, and stride speed but not VO2 Max, Neuro-FRS score, or timed 25-foot walking test. (Zesiewicz et al. 2018). RT001 was mostly safe with the most common adverse event being diarrhea.
RT001 (deuterated polyunsaturated fatty acid) // Infantile Neuroaxonal Dystrophy (INAD) // Phase 2/3
Infantile Neuroaxonal Dystrophy (INAD) is a rare genetic disease that causes progressive deterioration of the axons of the nervous system. It results in loss of control of the body, vision, and mental capacity. INAD is linked to a mutation in the PLA2G6 gene, which is responsible for breaking down certain fats.
INAD affects ~ 1 in 1,000,000 children. The onset of the disease begins in infancy. Life expectancy is 5 - 10 years. There are no FDA-approved treatments.
In one study, the loss of PLA2G6 gene was shown to be associated with mitochondrial dysfunction and elevated lipid peroxidation in fruit flies and human fibroblast cells. Deuterated polyunsaturated fatty acids were able to partially restore locomotor function in flies and mitochondrial function in human fibroblasts (Kinghorn et al. 2015).
In a small trial of two patients with INAD, deuterated linoleic acid was shown to be well tolerated and taken up by red blood cell membranes. Both patients saw improvements in INAD score ratings. No adverse events were reported. (Adams et al. 2019)
RT001 // Progressive Supranuclear Palsy (PSP) // Phase 2/3
PSP is a brain disorder that affects movement, speech, vision, and cognition. It usually leads to severe disability within 3 - 5 years of onset. It is associated with the degeneration of brain cells in the regions of the brain stem including the substantia nigra.
PSP affects ~1 in 16,000 people -- mostly over 60 years of age. There are no effective FDA-approved therapies for PSP.
The exact cause of PSP is not known. Possibilities include protein aggregates (microtubule-associated protein tau) and also elevated oxidative stress or lipid peroxidation (Odetti et al. 2000).
In April 2020, the FDA approved Retrotope to test RT001 in a Phase 2 / 3 trial for patients with PSP.
RT011 // Dry age-related macular degeneration (Dry AMD) // Pre-clinical
In July 2020, Retrotope announced the commencement of IND-enabling preclinical studies to use RT011 (another D-PUFA) to treat dry AMD.
Dry age-related macular degeneration is characterized by the loss of retinal pigment epithelial (RPE) cells and photoreceptors. The cause is not known but it has been linked to mitochondrial dysfunction, and several other mitochondria longevity companies are also choosing this disease as an indication.
RT011 is intended to stop lipid peroxidation in photoreceptor cells, which contain 15x more lipids than average cells. 50% of these photoreceptor lipids are DHA, which are especially prone to peroxidation.
D-PUFAs to treat other diseases:
There is preclinical evidence that deuterated polyunsaturated fatty acids may lower lipid peroxidation and mitigate cognitive decline in a mouse model of Huntington’s disease (Hatami et al. 2018)
Deuterated polyunsaturated fatty acids have been shown to mitigate nigrostriatal degeneration in mouse models of Parkinson’s disease (Shchepinov et al. 2011)
Deuterated polyunsaturated fatty acids have been shown to extend the lifespan of C. elegans by ~ 9% - 30% (Beaudoin-Chabot et al. 2019). I could not find any D-PUFA lifespan extension studies in mice.
Outlook for Retroptope
Data for their RT001 Phase 3 clinical trial for Friedreich’s Ataxia and also Phase 2/3 for Infantile Neuroaxonal Dystrophy expected in 2021.
My Thoughts
Retrotope’s approach is clever -- leveraging quantum mechanics with deuterium-infused salad dressing oil as a therapeutic. Less cool description: It’s slightly heavier-than-normal salad dressing molecules. Of all the peroxidation / ROS targeting companies this one seems like it has the most fundamental approach.
Mitotech
Funding Stage: Series A
Founded: 2010
Modality: small molecule drug, eye drop / oral
Founders/Team: Natalia Perekhvatova (CEO), Anton Petrov, Maxim Skulachev (CSO), Lawrence Friedhoff
Notable Investors:
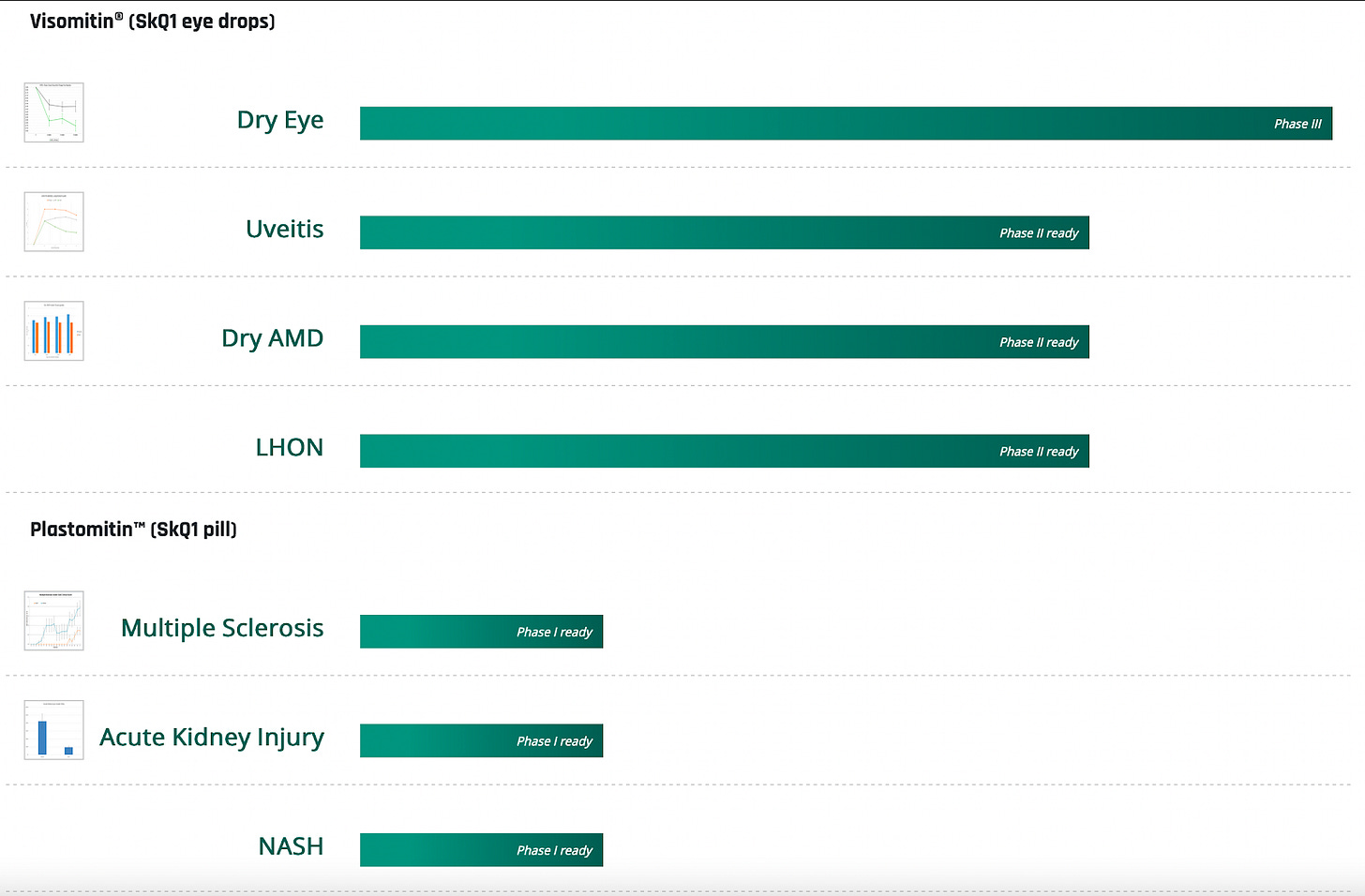
Pipeline:
SkQ1(Visomitin) // Dry Eye Syndrome // Phase 3
SkQ1(Visomitin) // Uveitis // Phase 2 (planned)
SkQ1 (Visomitin) // Dry age-related macular degeneration (dry AMD) // Phase 2 (planned)
SkQ1 (Visomitin) // Leber’s Hereditary Optic Neuropathy (LHON) // Phase 2 (planned)
SkQ1 (Plastomitin) // Multiple Sclerosis // Phase 1 (planned)
SkQ1 (Plastomitin) // Non-alcoholic steatohepatitis (NASH) // Phase 1 (planned)
SkQ1 (Plastomitin) // Acute Kidney Injury // Phase 1 (planned)
General Notes
Mitotech is a Luxembourg-based clinical-stage biotech company that develops SkQ1, a mitochondrial-targeting antioxidant to treat diseases associated with mitochondrial dysfunction.
Based on the research of Vladimir Skulachev, a Professor at Moscow State University in the early 200s.
SkQ1 is an antioxidant joined with a positively charged lipophilic molecule. This allows it to enter the inner mitochondrial matrix and scavenge Reactive Oxygen Species, thus preventing the peroxidation of cardiolipin -- an important lipid found in mitochondrial membranes.
Mitotech’s lead clinical program is an SkQ1 ophthalmic eye drop to treat Dry Eye Syndrome, an age-related disease.
Origins and Funding
Founded in 2010 in Russia, out of Moscow State University
Based on the research of Vladimir Skulachev.
Co-development of SkQ1 with Ora, an ophthalmology CRO. ~ $40M deal.
Pipeline Details
SkQ1 is Mitotech’s main asset -- a cardiolipin peroxidation inhibitor. It is administered as an eye drop solution (Visomitin) and also orally (Plastomitin).
SkQ1 consists of an antioxidant molecule linked via a hydrocarbon chain to a positively charged lipophilic molecule. This allows it to penetrate the inner mitochondrial membrane.
SkQ1 has been shown to prevent cardiolipin peroxidation in vitro (Skulachev et al. 2010). Cardiolipin is an important lipid found in the inner mitochondrial membrane (see Stealth BioTherapeutics).
SkQ1 (Visomitin) // Dry Eye Disease // Phase 3
Dry eye disease is a common disease caused by a lack of production of tears or deficient tear quality. If untreated it may lead to perforation of the cornea. Age is a risk factor.
Dry eye disease in older patients is usually caused by dysfunction of the meibomian gland that produces a lipid that prevents the evaporation of tears.
Phase 2 safety and efficacy trial showed a favourable safety profile with no adverse events. Statistically significant improvements in signs of dry eye including “corneal fluorescein staining and lissamine green staining in the central region and lid margin redness, and for the dry eye symptoms of ocular discomfort, dryness, and grittiness” (Petrov et al. 2016)
An earlier Phase 3 trial (VISTA-1) failed to meet its primary endpoint. The company followed with a pivotal Phase 3 trial currently being conducted (VISTA-2).
Already approved for use in Russia.
SkQ1 (Visomitin) // Uveitis // Phase 2 (planned)
Uveitis is a type of eye inflammation that can lead to blindness.
Uveitis can be caused by inflammatory disease, infection, injury, or autoimmune disease.
SkQ1 (Visomitin) // Dry age-related macular degeneration (dry AMD) // Phase 2 (planned)
Dry AMD is the leading cause of blindness in adults. It is characterized by degeneration of retinal pigment epithelial (RPE) cells and photoreceptors in the retina. Its exact cause is unknown but may be linked to dysfunctional mitochondria in RPE cells, which could lead to impaired nutrient and waste regulation (Kaarniranta et al. 2019).
SkQ1 (Visomitin) // Leber’s Hereditary Optic Neuropathy (LHON) // Phase 2 (planned)
LHON is a rare mitochondrial disease that can lead to blindness. It is caused by a mutation in the ND4 mitochondrial gene. (See GenSight Biologics GS0101)
SkQ1 (Plastomitin) // Multiple Sclerosis (MS) // Phase 1 (planned)
MS is a neurological disease caused by the immune system attacking neuron myelin. Possibly linked to mitochondrial dysfunction (Peixoto de Barcelos et al. 2019)
SkQ1 (Plastomitin) // Non-alcoholic steatohepatitis (NASH) // Phase 1 (planned)
A fatty liver disease that can cause cirrhosis, liver cancer, and liver failure.
70% of patients with Type 2 Diabetes have fatty liver disease. ~30% have NASH.
Many possible causes, mitochondrial dysfunction being one. (Caligiuri et al. 2016)
Outlook for Mitotech
Phase 3 trial for Visomitin to treat Dry Eye Syndrome completed enrollment in August 2020 with study completion expected end of 2020.
Mitotech to initiate a clinical development program in China.
Potential IPO filing in 2021, according to CEO Natalia Perekhvatova during a presentation at LSX 2020.
My Thoughts
Mitotech’s SKQ1 antioxidant is already commercially approved for dry eye in Russia. Perhaps not the best standard for regulatory approval but it’s a start. One can make the case that they have a platform of molecules (interchanging the lipophilic molecules and the antioxidant molecules), but I am generally not interested in ROS / peroxidation companies.
Other
Yuva Biosciences
Funding Stage: Seed
Total Funding: ?
Founded: 2017
Location: Birmingham, Alabama, USA
Modality: small molecule, cosmeceutical
Founders/Team: Keshav Singh
Notable Investors: Longevitytech.fund
Pipeline:
Drugs & cosmeceuticals // skin aging, hair loss // Pre-clinical
General Notes
Yuva Biosciences is an early-stage biotech company developing mitochondrial-targeted therapies or cosmetics to treat skin aging and hair loss.
Founded by Keshav Singh, Director of Cancer Genetics at the University of Alabama at Birmingham. Based on his lab’s demonstration of reversible skin aging and hair loss in a transgenic mouse model that had an inducible mutation in a gene responsible for mitochondrial DNA function.
Yuva is seeking to develop a natural compound or repurpose FDA-approved drugs to target skin aging and hair loss.
Origins and Funding
Yuva Biosciences was founded in 2017 by Keshav Singh, a cancer genetics researcher at the University of Alabama. Singh’s work in a transgenic mouse model of reversible mitochondrial dysfunction and skin aging/hair loss formed the basis of the spinout startup.
The chairman of Yuva, Greg Schmergel, is a serial entrepreneur who previously co-founded Nantero, a nanotech company.
Investors include Longevitytech.fund a Czech-based VC firm.
Yuva is a resident at Innovation Depot Inc, a startup space for tech companies located near the University of Alabama.
Pipeline Details

Drugs & cosmeceuticals // skin aging and hair loss // Pre-clinical
Skin wrinkling and hair loss are the most visible phenotypes of aging in humans. The global market for skin wrinkling was estimated to be worth $20B in 2018 according to Grandview research.
Yuva Biosciences is developing drugs and cosmeceuticals to treat skin wrinkling and hair loss based on Keshav Singh’s “mtDNA depleter mouse” model.
Singh’s group at UAB engineered a transgenic mouse that would express a POLG1 mutant gene only when given the drug doxycycline. POLG1 is a nuclear-encoded gene that is critical in the replication and repair of mitochondrial DNA. The triggering of the mutant POLG1 gene (“mtDNA depleter”) in mice resulted in decreases in mitochondrial DNA content in the heart, lung, brain, and liver. The mice also showed reduced levels of enzymatic activity of the electron transport chain complexes in the mitochondria. These mice also showed phenotypes associated with aging including gray hair, hair loss, progeroid head, slowed movement, and wrinkled skin, and skin inflammation. However, when doxycycline was halted and the mutant gene was switched off, it restored all of the youthful phenotypes (removed wrinkles, regrew smooth hair) and mitochondrial DNA content / enzymatic activity after one month (Singh et al. 2018).
There aren’t many details on Yuva’s development strategy. In a Forbes interview, Singh indicated that they were potentially looking to develop a natural product or repurpose FDA-approved drugs to restore mitochondrial function and reverse skin aging/hair loss.
My Thoughts
The science that Yuva Biosciences is based on is fascinating. While it is fairly trivial to cause mice to age faster, it is much more impressive to make this aging process reversible as in their 2018 paper. The mouse model they have developed can be used to investigate downstream pathways to target with drugs, though you can debate whether this is a good model of aging.
The company is still in the early stages and there is not much public information on their development timeline or exactly how they will translate their discovery. Suffice to say the markets they are targeting (anti-aging cosmetics and hair loss) are gargantuan. We need to recognize that these kinds of products get many people more excited about longevity than, say, drugs that target NASH.
Guided Clarity
Funding Stage: Seed
Total Funding: ?
Founded: 2018
Location: San Francisco, California, USA
Modality: Medical food, protein-peptide, oral
Founders/Team: Helen Chen, Olof Mollstedt
Notable Investors: IndieBio
Pipeline:
Medical food (alpha-lactalbumin-oleic acid) // Cancer, inflammation, age-related disease // Pre-clinical
General Notes
Guided Clarity is a pre-clinical stage biotech company developing alpha-lactalbumin a natural product/medical food found in human breast milk to treat metabolic dysfunction in cancer, inflammation, and aging.
Founded by Helen Chen, formerly a researcher at Texas A&M and COO of Ambryx, and Olof Mollestedt, formerly CEO of Ambryx. Ambryx previously investigated the therapeutic use of milk proteins.
Guided Clarity was funded/incubated at IndieBio.
Pipeline Details
Medical Food (alpha-lactalbumin) // Metabolic dysfunction, cancer, inflammation, aging // Pre-clinical
Guided Clarity is developing a medical food whose active ingredient is alpha-lactalbumin, a 123-amino acid protein found in milk that is enzymatically broken down into bioactive peptides in the body. The company believes these peptides play a role in the signaling of mitochondrial turnover and renewal. Target therapeutic indications include metabolic dysfunction, cancer, and aging (osteoarthritis).
Peptides derived from proteins found in milk (such as alpha-lactalbumin, lactoferrin, etc) may have anti-cancer properties through a number of proposed mechanisms. Several studies demonstrate that these peptides kill tumors in vitro. (Chen et al. 2014).
In 2011, Guided Clarity’s co-founders conducted studies on a cow’s milk peptide product (AminoAct). It was shown to induce apoptosis of colon cancer cells in vitro and slightly increase the lifespan of C. elegans. It also was shown to be safe to consume in a Phase 1 placebo trial of healthy human volunteers (Kreider et al. 2011).
Some in vitro studies suggest that alpha-lactoalbumin complexes can induce macroautophagy (recycling of mitochondria, and other organelles and proteins) within the cell (Aits et al. 2008). There is also a link between alpha-lactalbumin and mitochondrial uncoupling, which could be beneficial for metabolic disease (Kohler et al. 2001).
The company is gearing up for clinical trials and scaling of their manufacturing facility this year (according to an interview in 2019). Although alpha-lactoalbumin is found in high levels in breast milk, it is also found in cow's milk, which will be the source for Guided Clarity’s product.
My Thoughts
I had trouble finding in vivo studies linking alpha-lactalbumin and restoration of mitochondrial function. Natural medical foods are favourable from a safety standpoint but more efficacy data is needed.
Summary and Final Thoughts
Mitochondria are complex. It’s still an open question on how they drive aging. Hallmarks of Mitochondrial Aging? Mitochondria have their own DNA, membranes, ribosome, move around (sometimes outside of the cell!), undergo fission and fusion, and provide one of the most critical cellular functions. Since they are almost like their own organism they probably deserve their own “Hallmarks of (Mitochondria) Aging” paper. But just like the original Hallmarks paper, it will be filled with many correlations and questions while causation is not always clear.
Mitochondrial transfer is very promising. I’ll admit I am biased towards replacement therapies for anti-aging when it makes sense. It’s a clean philosophical approach that lends itself to engineering more than traditional drug development. And unlike stem cell therapies or cellular transplants (Lineage Cell Therapeutics) mitochondria can be replaced easily without the need for extracellular scaffolding or any kind of extra in situ differentiation. There will likely be a number of challenges to work out, though (sourcing allogeneic mitochondria, systemic distribution, determining long-term side effects, etc).
One caveat: It is possible that transplanting healthy mitochondria into an old and dysfunctional cellular environment will quickly impair the transplanted organelles. Also, need to consider the microtubules mitochondria use to move around the cell.Pro-sports mitochondrial transfer? Mitochondria density in skeletal muscle declines with age. It makes sense that aging professional athletes might be some of the first people in line to get mitochondrial transfer or mitochondrial biogenesis treatments. How much is one more year of high-level performance worth to Lebron James?
Protect mtDNA or remove mutations? GenSight Biologics is definitely one of the most interesting biotech companies I have ever stumbled upon. And while their therapies aren’t considered anti-aging at the moment (save for GS020 for dry AMD), the technology is a step towards possibly solving the problem of mitochondrial mutations. But even here it is not clear whether it is more important to protect mtDNA from new mutations or stop already mutated mtDNA from accumulating via clonal expansion (like Shift Bioscience). Some researchers have also proposed using various DNA editing techniques (TALENS, Zinc Finger Nuclease, CRISPR) to destroy mutant mitochondrial DNA.
Clinical trials outlook. Seven mitochondria longevity companies are currently in clinical trials. The immediate significance of the outcome of these trials for treating broader aging varies. We aren’t going to be using GenSight’s gene therapy for mtDNA-mutation-related aging anytime soon despite its imminent approval. However, I believe the therapies that can be translated to treat sarcopenia (perhaps Epirium Bio’s (+)-epicatechin, mitochondrial transfusion) have the best chance of being adopted by the general public in the near future.
Longevity is moving FAST. In the several weeks in between when I started researching mitochondria longevity companies and when I “finished”, several new papers directly related to the science of some of these companies were published and some companies announced major business developments (ex. Mitokinin x Abbvie). This just serves to highlight how quickly things are moving in the longevity industry. And by building, investing, or championing this cause you can help it move A LOT faster.
*This week’s newsletter special thanks go to Daniel Ives and Robert Ziman.
Tweet of the Week
Crypto has unlocked a once-in-a-generation transfer of wealth to a cohort of technologically progressive risk-takers. Think bigger.
“Longevity, not Lambos.”
-NATHAN