#022: A Map of Mitochondria Longevity Companies (PART 1)
Mitochondria x Longevity. BioAge Phase 2. Longevity SPACs.
📡In this edition of Longevity Marketcap Telemetry
Last Week in Longevity
Longevity Futures
Longevity Jobs
A Tour of All Mitochondria Longevity Companies
Tweet of the Week
Dear Longevity Nerds: 🎉🎉🎉We have reached 925 subscribers -- still doubling every ~7 weeks. The movement is growing stronger and stronger every day thanks to early adopters like you! Spread the word about longevity biotechnology and we can solve aging even faster.
Note: Sorry this week’s newsletter is a bit long (14,000+ words, R.I.P. your inbox). Future newsletters will be much shorter now that this particular topic is out of the way.
Edit: I broke Substack — I am forced to split the newsletter into Part 1 and Part 2.
-NATHAN
Disclaimer: None of this should be taken as financial advice. This information is for educational purposes only.
📝Last Week in Longevity
BioAge enters the clinic with Phase 2a trial. Kristen Fortney’s a16z-backed AI/ML drug discovery startup announced it had initiated a Phase 2a clinical trial of BGE-117 to treat unexplained adult anemia (UAA), an age-related disease that affects 10% of those over 65 years of age. Topline data is expected in H1 2022.
BGE-117 is a Hypoxia-Inducible Factor (HIF) activator licensed from Taisho Pharmaceuticals that has been shown to increase Hb and EPO levels in humans without UAA in Phase 1 trials. BGE-117 may also have benefits that extend to broader aging: BioAge mined proprietary biobanks to determine that individuals that had higher levels of HIF were much more likely to live past 85. A success here would not only be huge for the longevity industry but also AI/ML drug discovery as a whole. Truly exciting.
Christian Angermayer’s Apeiron SPAC -- David Sinclair and Peter Attia to be co-chairmen. Angermayer’s Frontier Acquisition Corp, a blank-check SPAC company, will list as early as March. Angermayer is also a founder and financier of Cambrian Biopharma, a multi-portfolio longevity biotech company that came out of stealth this month. Peter Attia, a medical doctor with a longevity practice and popular podcast, is well known in the longevity/biohacking space (as is David Sinclair). I’m guessing this is a nutraceuticals or wellness play and not a deep biotech acquisition.
ChromaDex (NYSE:CDXC) stock surges over 100% on Phase 3 COVID recovery trials. ChromaDex is the maker of TruNiagen, a nicotinamide riboside (NR) NAD+ boosting supplement. In a preprint posted on medrXiv, the company showed that treatment of N-acetylcysteine, L-carnitine tartrate, nicotinamide riboside, and serine + standard of care (hydroxychloroquine) was able to reduce recovery times of mild to moderate COVID patients by ~38% (versus standard care) in a double-blind trial. In addition to news of a $25M private share sale, the price of ChromaDex’s shares has surged nearly 100% over the week.
Humacyte to go public via SPAC (NASDAQ:AHAC). It’s raining longevity SPACs and IPOs these days. Humacyte, a tissue engineering company founded by Laura Nikalson (Yale), has developed a cell-free artery replacement that is currently being tested in late-stage clinical trials. The deal will value Humacyte at $1.1B.
📅Longevity Futures
Joao Pedro de Magalhaes (Professor @ University of Liverpool, Centaura) on the Longevity Biotech Show on Clubhouse. March 4th, 1 PM PST / 4 PM EST. Robert Ziman and I are hosting renowned aging researcher Joao Pedro de Magalhaes on our weekly Longevity Biotech Show on Clubhouse this week. Stick around at the end for a casual “after-party” longevity biotech chat/networking.
Previous chats had an amazing number of people from academia and industry drop in including (but not limited to):Alexandra Bause (Apollo), Scott Schandler (Longevity Biotech), Joshua Elkington (Axial), Ethan Perlstein (Perlara), Andrew Brack (UCSF), Matt Scholz (Oisin Biotechnologies), Ryan Bethencourt (Wild Earth, IndieBio), Jordan Miller (Volumetric), Keith Comito (Lifespan.io), Jean Hebert (Albert Einstein College of Medicine), Daniel Ives (Shift Biosciences), among others.
The Longevity Biotech Show Schedule (1 PM PST / 4 PM EST):
March 4th, 2021. Joao Pedro de Magalhaes (University of Liverpool, Centaura)
March 11th, 2021. Keith Comito (Lifespan.io)
March 18th, 2021. Aubrey de Grey (SENS Research Foundation)
Longevity Leaders 2021 Online Conference May 4th - May 7th, 2021: An industry and academia conference with networking events. Basic passes are free but access to premium online events is paid. Early bird discounts are also available. A great lineup of speakers: Aubrey de Grey, Nir Barzilai, Joao Pedro de Magalhaes, Sergey Young, Ronjon Nag, representatives from Alkahest, Stealth BioTherapeutics, Navitor, and many more.
The Business of Biotech - RA Capital Discussion Session, March 10th, 17th, 24th, 2021. Peter Kolchinsky’s RA Capital has a free course on the business side of biotech and twice a year they have a live discussion session on the material.
Featured Longevity Jobs
If you are hiring send me a link to your job postings and I will post them on longevitylist.com (it’s free).
Looking for more jobs, companies, or investors in the longevity biotechnology industry? Check out my website LongevityList.com.
A Tour of All Mitochondria Longevity Companies
In this newsletter, we’re going to take a look at all the longevity biotech companies that are developing therapies that target mitochondrial dysfunction -- one of the “Hallmarks of Aging”.
Mitochondria companies make up one of the biggest subcategories in longevity biotech. This makes sense as mitochondria are an extremely critical component of our cells.
There are ~20+ longevity mitochondria companies currently, ranging from early stage-startups to Nasdaq-listed public companies. From small molecule drugs to gene therapies and mitochondrial transfusions. Seven of the companies are in clinical trials TODAY. (Check out my longevity clinical trial tracker!)
What are the most promising companies? Let’s find out.
Spoiler: I like Cellvie, Minovia, Shift Bioscience, Mitokinin, CohBar, and Epirium Bio. Maybe Mitrix.
List of mitochondria aging companies by subcategory:
Mitochondrial Transfer
Minovia: Mitochondria-infused stem cells to treat rare mitochondrial mutation diseases.
Cellvie: Mitochondrial transfusion for ischemia-reperfusion injury, also plans for aging.
Mitrix: Mitochondrial transfusion for aging.
See this exceptional review (Liu et al. 2021) on the therapeutic use of mitochondrial transfer. It has beautiful tables.
Mitochondrial Mutations
GenSightBiologics (EPA:SIGHT): Gene therapy to cure diseases that cause blindness by expressing a mitochondrial gene in the nucleus (allotopic expression).
Shift Bioscience: Drugs that shift the ratio of mutated to normal mitochondrial.
Mitochondria and Metabolism
CohBar (NASDAQ: CWBR): Mitochondrial-derived peptides to treat non-alcoholic fatty liver disease and other diseases.
Pano Therapeutics: Targeting mitochondrial ion channels and transporters. Developing a superior version of metformin.
Continuum Biosciences: Mitochondrial uncoupler drugs that increase energy expenditure to treat metabolic disease.
Equator Therapeutics: YC-backed company developing a mitochondrial uncoupler drug to treat obesity and type 2 diabetes. Nature paper: (Bertholet et al. 2019).
Mitochondria Quality Control / Biogenesis
Epirium Bio: (+)-Epicatechin synthetic flavanol to stimulate mitochondrial biogenesis.
Mitokinin: Small molecule drugs that increase the activity of the active form of PINK1, a regulator of mitochondria quality control via mitophagy.
712 North: Personalized mitochondrial drugs based on OMA1-OPA1 pathways that control mitochondrial dynamics (fission/fusion) and apoptosis.
Peroxidation / Oxidation
Stealth BioTherapeutics (NASDAQ:MITO): Elamipretide peptide to stabilize cardiolipin and reduce peroxidation of mitochondrial lipids.
Retrotope: Deuterated polyunsaturated fatty acids to stop mitochondrial membrane lipid peroxidation.
Mitotech: Antioxidant that can be transported into mitochondria to reduce oxidative damage.
Other
Yuva Biosciences: Developing cosmeceutical and pharmaceuticals to reduce wrinkles in skin and hair loss by using a mtDNA mutation model.
Guided Clarity: Developing a natural compound found in mother’s milk that rejuvenates cells by targeting mitochondria.
A brief background on mitochondria
Mitochondria are the powerhouse of eukaryotic cells. Their main function is to convert the energy in the chemical bonds of glucose into ATP (adenosine triphosphate), the universal energy currency of all living things. Mitochondria are also responsible for beta-oxidation regulation of fatty acid metabolism, calcium homeostasis, and apoptosis (cell suicide).
Mitochondria Specs:
~ 1 micron across (which is roughly 10,000 atoms)
They have their own DNA --
37 genes, 13 of which are protein-encoding.
Circular double-stranded DNA package.
~16.6 kilobases.
Mitochondrial DNA (mtDNA) is generally inherited maternally
Made of ~1000 different proteins.
They have an inner and outer membrane.
Roughly 100 - 1000 mitochondria per mammalian cell.
Highly dynamic. Constantly divide (fission) or fuse with other mitochondria and move around in a cell via microtubules.
Capable of migrating from cell to cell.
Likely evolved from ancient bacteria that got stuck inside one of our single-celled ancestors (“endosymbiosis”).
And most importantly:
Mitochondria perform oxidative phosphorylation to make ATP: Coenzymes produced in the citric acid cycle (NADH, FADH2) deposit their electrons into the electron transport chain, a series of “Complexes” (I, II, III, IV) in the inner mitochondrial membrane. This releases energy that is used by the Complexes to pump protons out of the inner matrix, forming a gradient. Protons flow back into the inner matrix and turn a turbine-like structure called ATP synthase, which makes ATP. (Video)
Mitochondria and aging
Mitochondrial dysfunction has been identified as one of the nine “Hallmarks of Aging” in the often-cited review paper of the same name. Researchers think dysfunctional mitochondria might play a causative role in aging but the “how” is still up for debate.
Here’s a list of aspects of mitochondria that change with age that might be a cause of aging (all of these are controversial):
Decrease in energy production and oxidative phosphorylation:
Elderly individuals were found to have~40% lower levels of mitochondrial oxidative and phosphorylative activity in skeletal muscle cells compared to young controls, as measured by in vivo 13C/31P NMR spectroscopy. (Petersen et al. 2003).
Increase in reactive oxygen species (ROS) production and oxidative damage (***Read this review if you love tables! → Shields et al. 2021***):
Reactive oxygen species (ROS) are highly reactive molecules that contain oxygen (hydrogen peroxide, hydroxyl ion, superoxide anion, etc). In the mitochondria, ROS are formed when electrons leak from the electron transport chain.
ROS and oxidative damage increase with age, which led some to believe that ROS was a leading cause of aging. In associative studies, ROS has been correlated with age-related diseases and lifespan in long-lived animals and people. But the results from experimental manipulation of ROS are much more complicated and conflicting. (Lots of variation in results: How was ROS measured? In vitro or in vivo? What species of ROS? ROS location? What stage of life? Which model organism?) (Sanz 2016 )
Some studies show that increasing ROS can actually extend lifespan in some model organisms. This suggests that at low levels ROS signals for protective stress response, but at high levels or in damaged states ROS can overwhelm cells (reviewed in Hekimi et al. 2011).
Increase in mitochondrial DNA mutations:
Mitochondrial DNA mutations accumulate ~15x faster than nuclear DNA mutations (Allio et al. 2017). A cell can have a mix of mutated and non-mutated mitochondria (“heteroplasmy”).
One study suggested that a threshold of ~70%+ heteroplasmy is needed to have a phenotypic/pathogenic effect (at least in mtDNA mutation diseases), which is higher than what can likely be achieved by aging (Rossingol et al. 2003).
Most age-related diseases (Alzheimer’s, Parkinson’s, cancer, heart failure, diabetes, and sarcopenia) are correlated to mtDNA mutations. Some studies suggest that the accumulation of age-related mtDNA mutations is driven by clonal expansion of mutated mitochondria over time rather than random acute mtDNA damage. (reviewed in Chocron et al. 2019.)
Age-related accumulation of specific mitochondrial mutations is correlated to aspects of aging including decline of cognitive and physical function and all-cause, dementia, and stroke mortality (Tranah et al. 2018).
Impaired mitophagy/quality control:
Mitophagy is the process of removal of damaged mitochondria by autophagosomes within the cell (mediated by PINK1 and Parkin pathway, FUNDC1, etc) (Chen et al. 2020).
Studies suggest that levels of mitophagy decrease with age and that impaired mitophagy is linked to neurodegenerative diseases (reviewed in Bajula et al. 2020).
Decreased mitochondrial biogenesis:
Older individuals have lower mitochondrial density in skeletal muscle compared to the young (Crane et al. 2010).
Transgenic male mice overexpressing PGC1α, a protein associated with mitochondrial biogenesis, are protected from sarcopenia in old age. (Yang et al. 2020)
Altered mitochondrial dynamics (reviewed in Sharma et al. 2019):
In general, mitochondrial fission and fusion alone are not always clearly pro or anti-aging. It often depends on the tissue in question and the stage of life of the organism.
Fission in midlife seems to facilitate mitophagy (good). Fusion seems to be associated with lifespan extension in C. elegans but not fruit flies.
Mitochondria can move around inside the cell via microtubules (trafficking). In neurons, trafficking decreases with age.
Tldr; Everything is still an open question. Aging biology is complex-- deal with it.
Target Diseases for Mitochondria Longevity Biotech Companies
Aging is not an accepted clinical indication (yet).
So the current playbook for longevity biotech companies is to target a disease that shares the same underlying cause as one of the hallmarks/targets of aging for their first clinical trials and expand from there. Usually, this means an age-related disease or a rare genetic disease.
Mitochondrial dysfunction is implicated in many diseases of aging (Haas et al. 2019). Longevity companies targeting mitochondrial dysfunction generally choose clinical indications such as:
Primary Mitochondrial Diseases caused by mtDNA mutation or nuclear DNA mutations (LHON, Pearson syndrome, etc)
Muscle dystrophy diseases or muscle loss (Duchenne muscular dystrophy, Becker, sarcopenia)
Metabolic disorders (NASH, Obesity, NAFLD, Type 2 Diabetes)
Neurodegenerative disease (Alzheimer’s, Parkinson’s, etc)
Diseases linked to ROS oxidative damage (Ischaemia-reperfusion injury)
Mitochondrial Transfer
Minovia
Funding Stage: ?
Total Funding: $10M+
Founded: 2012
Location: Haifa, Israel
Modality: mitochondrial transfer
Founders/Team: Natalie Yivgi-Ohana, Uriel Halavee, Ephraim Aharonson
Notable Investors:
Pipeline:
Links
General Notes
Minovia is a clinical-stage company based in Israel that develops mitochondrial transplantation/transfusion to treat rare mitochondrial diseases caused by mtDNA mutations. They are the first company to perform this kind of therapy in human trials.
Minovia’s technology, Mitochondrial Augmentation Therapy (MAT), involves the enrichment of autologous hematopoietic stem cells with donor mitochondria followed by reinjection into the patient.
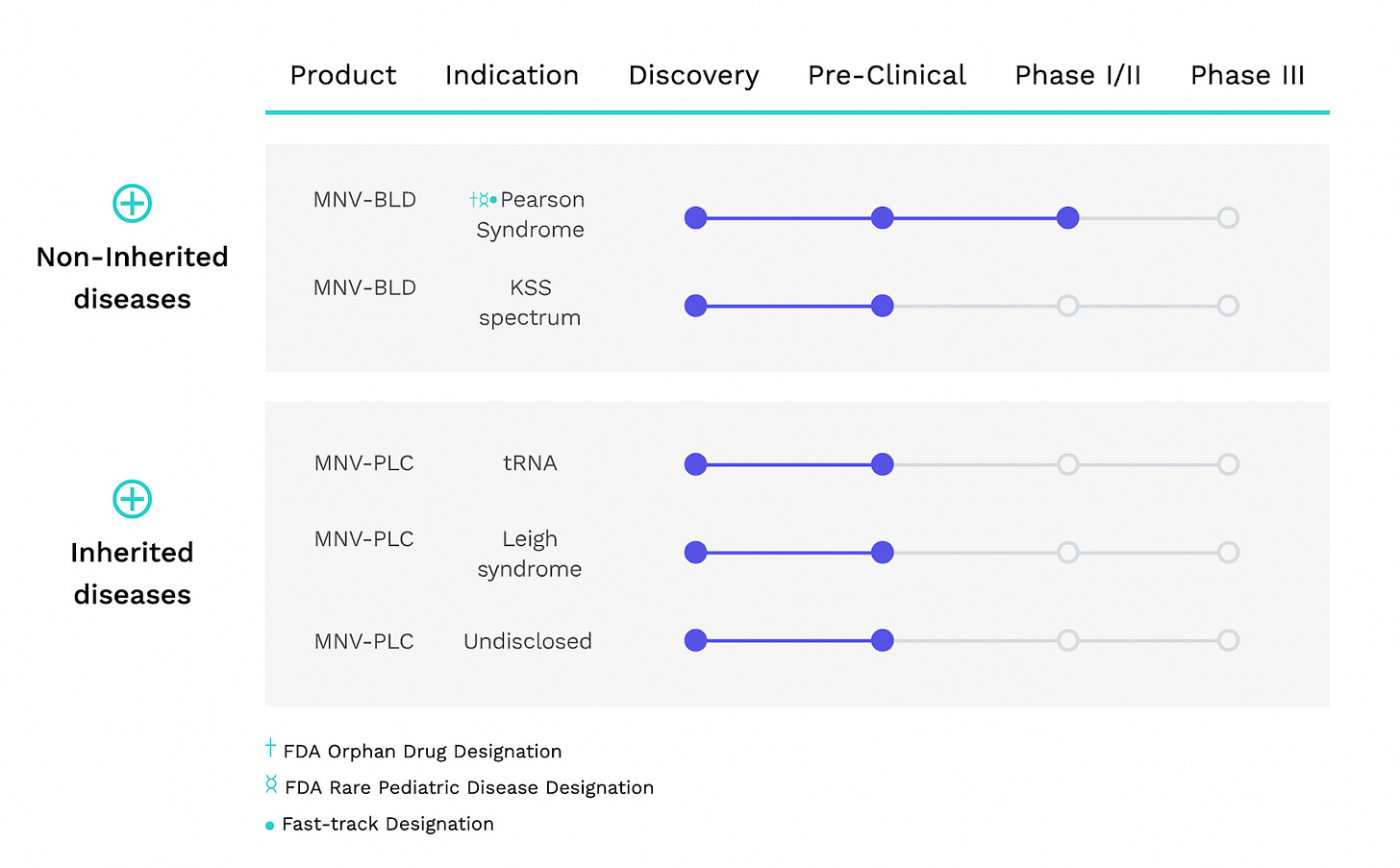
Minovia is currently in Phase 1/2 trials for Pearson’s Syndrome, a rare non-inherited mitochondrial disease. Minovia has also initiated Phase 1 trials for Primary Mitochondrial Disease.
An early compassionate use study of Minovia’s MAT to treat three Pearson Syndrome patients showed promising improvements in quality of life and function.
Origins and Funding
Minovia was founded in 2011 by Natalie Yivgi-Ohana (CEO), a former postdoc at the Weizmann Institute in Israel, Uriel Halavee, and Ephraim Aharonson.
Minovia is based in Israel but also has offices in Cambridge, Massachusetts.
Continuing in the footsteps of the first artificial mitochondrial transfer demonstrated by Clark et al. in 1982.
Initiated clinical trials for Pearson Syndrome after results from compassionate use of their therapy in three patients at Sheba Medical Center in Tel Aviv.
Last funding round: $10M
Pipeline Details
MAT (Mitochondrial Augmentation Therapy) // Pearson Syndrome // Phase 1
Pearson syndrome is an ultra-rare mitochondrial disease characterized by bone marrow that produces ring-like premature red blood cells (sideroblasts) and pancreas dysfunction. It is caused by deletions in mitochondrial DNA during development or in the egg.
Pearson syndrome is usually fatal in infancy. Only 100 cases are recorded in the medical literature. There are no FDA-approved treatments.
Minovia’s MAT for non-inherited diseases takes a patient’s hematopoietic stem cells (blood-forming stem cells identified by CD34 marker) and enriches them with functional mitochondria from the mother. The stem cells are then injected back into the patient with the hopes the mitochondria will transfer horizontally to other cells. Roughly 1 - 3 million mitochondria are transferred in a process that takes 40 hours from vein-to-vein.
Early evidence of Minovia’s MAT efficacy in a small compassionate use study showed that two out of the three patients in the study saw increases of 40% - 150% wild-type mitochondrial DNA relative to baseline 3+ months after therapy. Metabolic function of white blood cells, aerobic ability, fine motor control, and quality of life (IPMDS score) saw improvements. There were no major adverse events other than those related to the patient blood draw/apheresis (anemia, etc) (Jacoby et al. 2018).
Phase 1 clinical trial for Pearson’s syndrome expected completion in 2021.
MAT (Mitochondrial Augmentation Therapy) // Primary Mitochondrial Disease (PMD) // Phase 1
Primary Mitochondrial Disease (PMD) refers to a heterogeneous group of diseases caused by inherited genetic mutations (nuclear or mitochondrial) that result in mitochondria with impaired energy production. PMD affects 1 in 4300 live births.
In diseases caused by inherited mitochondrial mutations, the mother’s mitochondria cannot be used for donation in MAT. For these patients, allogeneic mitochondria are harvested from the placentas of unrelated donors.
Minovia is currently recruiting patients for a small 6-person Phase 1 study of MAT to treat PMD. Expected primary study completion in 2022.
Other trials of MAT and pre-clinical data
In a single case of compassionate use, Minovia’s MAT improved function in a patient with Kearns-Sayre syndrome (Results: weight gain, cessation of seizures, regained the ability to sit, walk, and speak) (Yosef et al. 2020). Kearns-Sayre syndrome is a rare mitochondrial disease.
In one study, young mitochondria transferred to old mice were able to improve memory function of the old mice (water maze test), decrease ROS production and increase mitochondrial enzyme activity in the brain, and improve muscle function (+100% in a forced swimming test times). Note the total doses were relatively high (5 million per mouse). (Zhao et al. 2020). This appears to be the only published mouse study of mitochondrial transfer to treat aging.
Outlook for Minovia
Phase 1 clinical trial for Pearson’s syndrome expected completion in 2021.
Phase 1 clinical trial for Primary Mitochondrial Disease expected primary completion in 2022.
My Thoughts
I am extremely interested in mitochondrial transfer therapies and will be watching Minovia closely since they are the furthest along in clinical development. Philosophically, replacement therapies are very clean.
However, the technology is still being developed and there are many outstanding questions (long-term safety, biodistribution, effect duration, etc) Is there a limit to how many mitochondria you can transfer? The biggest question may be the duration of any rejuvenation effect when young mitochondria are placed in a hostile aged-cell environment. This is not an issue for the rare mitochondrial mutation diseases they are targeting but something to think about for the future. Note that Minovia does not even mention age-related disease in its pipeline roadmap, but this seems like a no-brainer expansion.
Cellvie
Total Funding: $5M
Founded: 2018
Location: Zurich, Switzerland
Modality: Mitochondrial transfer, organelle therapy
Founders/Team: Alexander Schueller, James McCully, Pedro del Nido, Sitaram Emani
Notable Investors: Kizoo Technology Capital
Pipeline:
Autologous Mitochondria Transfer // Ischemia-reperfusion injury (in kidney transplants) // Pre-clinical
Links:
General Notes
Cellvie is a preclinical-stage company developing autologous mitochondria transfer to treat ischemia-reperfusion injury (IRI) -- tissue damage when oxygenated blood supply returns after a period of oxygen restriction. IRI usually arises during heart attacks, strokes, organ transplants, and heart surgeries. Cellvie plans on eventually extending their mitochondrial transfer technology to treat aging / age-related disease, likely using an allogeneic source of mitochondria.
The company is a spinout from Harvard where some of the co-founders developed a method of mitochondria extraction and purification that could be performed in 30 minutes. Numerous animal studies and a small ongoing pilot clinical trial for cardiac ischemia-reperfusion injury suggest that the technology is safe and able to restore organ function. Cellvie’s first clinical trial will be for IRI in kidney transplant surgery.
Backed by Michael Greve’s Kizoo Technology Capital, a German VC firm that specializes in longevity biotechnology.
Origins and Funding
Cellvie is a spinout founded by medical researchers at Harvard. The company’s founders have been developing their mitochondria transfer technology for ischemia-reperfusion injury (IRI) since 2009. Previously they had been researching pharmacological therapies for IRI, which had limited success.
Alexander Schueller (CEO) previously founded Adhesys, which was acquired by the Gruenenthal Group. James McCully (co-founder) invented mitochondrial transfer for IRI at Harvard.
In January 2021, Cellvie raised $5M in seed funding from Kizoo Technology Capital (founded by Michael Greve), which has backed many other notable longevity biotech companies.
Pipeline Details
Autologous Mitochondria Transfer // Ischemia-reperfusion injury (IRI) in kidney transplantation // Preclinical
Ischemia-reperfusion injury (IRI) is tissue damage caused by the resupply of oxygenated blood after a period of blood supply restriction. It occurs during organ transplants, heart attacks, and strokes.
The pathophysiology of IRI is complex. At a high level, biochemical processes in the mitochondria are disrupted due to lack of oxygen and when oxygen is reintroduced it causes harmful reactions: High levels of ROS production and mitochondrial swelling from calcium ion overload lead to mitochondrial damage and potentially cell death. (IRI also has an immune system response component.)
IRI plays a major role in the non-function or delayed function of kidneys after transplantation, which can occur in up to 50% of transplants. Long-term damage stemming from IRI can lead to outright rejection of the transplanted organ.
Cellvie’s mitochondrial transfer therapy attempts to address the mitochondrial damage caused by IRI by augmenting cells with healthy mitochondria sourced from the patient.
Cellvie’s founders have developed a process of rapid isolation and purification of mitochondria using a commercial tissue dissociator and differential filtration. The process is capable of yielding 20 billion viable mitochondria from a 0.18 g tissue sample in 30 minutes (Preble et al. 2014). Traditional methods of mitochondrial extraction using centrifuges can take up to 120 minutes. A short processing time is crucial for emergency medicine (stroke, heart attack) and maintaining mitochondria viability. The extracted mitochondria are typically suspended in a delivery vehicle and injected directly or transfused through the bloodstream.
A pilot study of using Cellvie’s therapy to improve cardiac function of children with IRI after undergoing heart surgery saw no short-term adverse events. Improved cardiac function was seen in four out of the five patients within 48 hours after treatment (Emani et al. 2018). Typically only 29% of patients with similar conditions (on extracorporeal membrane oxygen) see recovery.
In animal studies with pigs, Cellvie’s autologous mitochondrial transfer was superior in preventing kidney necrosis and in restoring filtration function versus controls. There were no adverse events (Doulamis et al. 2020).
Outlook for Cellvie
Cellvie intends to initiate clinical trials using mitochondrial transfer to treat kidney transplant patients. Future applications include myocardial ischemia, ischemic stroke, and aging.
Cellvie plans to commercialize their mitochondrial transfer technology by 2026.
My Thoughts
By developing a fast extraction technology Cellvie has carved out a niche in mitochondrial transfer therapy for use in emergency medicine ischemia-reperfusion injury (so niche!). And because IRI is an acute condition, Cellvie's mitochondria don’t have the same viability/duration requirements as they would for a genetic disease or aging. Ideally, the therapy will work for aging too (see mouse aging study), but if not at least investors will have an initial indication that has a reasonable chance of success. Cellvie doesn’t use cells as the delivery vector for the mitochondria (unlike Minovia, which uses autologous hematopoietic stem cells) so biodistribution could be an issue.
Mitrix
Funding Stage: Seed
Total Funding: 250k
Founded: 2018
Location: Pleasanton, California, USA
Modality: Mitochondria transfer
Founders/Team: Tom Benson
Notable Investors: R42 AI and Longevity Fund, Longevitytech.fund
Pipeline:
Whole-body mitochondrial transfusion // Alzheimer’s, Parkinson’s, heart disease, sarcopenia // Preclinical
General Notes
Mitrix is an early-stage biotech company developing whole-body mitochondria transfusion to treat age-related disease. Mitochondria will be grown in stem cell bioreactors.
Founded by Tom Benson, a serial entrepreneur and founder of Timepoint Capital. Collaborators include University of Manitoba Professor Benedict Albensi, and Professors Alexander Rabchevsky, Patrick Sullivan, and Samirkumar Patel from the University of Kentucky.
Investors include R42 (Ronjon Nag’s fund) and Longevitytech.fund
Planning a pre-clinical demonstration of the technology in animal models.
My Thoughts
Unfortunately, there isn’t much public information about this company right now. As another mitochondria transfer company, Mitrix will be competing against Minovia and Cellvie (see above for more background on the technology), who appear to be further ahead in development.
Mitochondrial Mutations
Shift Bioscience
Funding Stage: Seed
Total Funding: ?
Founded: 2017
Location: Cambridge, UK
Modality: small molecule drugs
Founders/Team: Daniel Ives
Notable Investors: Johnathan Milner
Pipeline:
SB002 // MELAS (rare mitochondrial disease) // Pre-clinical
Links
General Notes
Shift Bioscience is an early-stage pre-clinical company founded by Daniel Ives (an all-around cool dude) based on his doctoral research at Cambridge/Crick Institute on small molecules that can shift the balance between the number of normal versus mutated mitochondria in cells.
Shift Bioscience’s candidate molecule (SB002) works by enabling healthy mitochondria to outcompete mutated mitochondria for protein resources thus lowering the relative population of dysfunctional mitochondria.
The company’s initial indication will be in a rare mitochondrial disease (MELAS) but has plans to expand to age-related diseases.
Origins and Funding
Shift Bioscience’s scientific basis follows results from Ian Holt (Ives’ Ph.D supervisor) in the 90s that showed a cancer cell fused with an enucleated cell that had a MELAS mtDNA mutation could cause a change in the balance of healthy versus dysfunctional mutated mitochondria (heteroplasmy).
Ives used bioinformatics approaches to discover pathways that were associated with this shift in the percentage of mutated mitochondria and discovered a small molecule drug could modulate this shift in their cell model.
Shift Bioscience was originally bootstrapped by Daniel Ives with his own money and money from friends/family. The initial experiments were conducted in a “virtualized” manner through contract research organizations (CROs).
The company got its first outside investor funding from Jonathan Milner, a life science entrepreneur-investor who co-founded Abcam. Shift also received additional seed funding in 2019 and 2021.
Pipeline Details
SB002 // MELAS (rare mitochondrial disease) // Pre-clinical
MELAS (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes) is a rare mitochondrial disease that is distinguished by stroke-like episodes and buildup of lactic acid in the blood. MELAS also involves progressive impairment of motor skills, vision, and cognitive ability. It is caused by a mutation in mitochondrial DNA (usually in the MT-TL1 gene), usually the m.3243A>G point mutation (requires 70%+ heteroplasmy).
The onset of MELAS usually begins in childhood and affects ~ 16 in 100,000 people. The median survival time from diagnosis is ~17 years. There are no FDA-approved treatments specifically for MELAS.
Ives’ doctoral research investigated how cancer cells (A549) fused with a myoblast with the MELAS mitochondrial DNA mutation (and nucleus removed) could cause the percentage of mutated mitochondria in the cell to decrease in some of the cells (Ives 2013). Using bioinformatics tools (enrichment maps that cluster genesets by functional themes) he deduced that endoplasmic reticulum stress response and amino acid starvation may be responsible for the change in percentage of mutant mitochondria of the cells. Ives further demonstrated that 2-deoxy-D-glucose, a molecule known for inducing endoplasmic stress response, could decrease the percentage of mutant mitochondria of the cells.
Shift is developing SB002, a drug that has similar mutant mitochondria decreasing effects as 2-deoxy-D-glucose but less toxic. In unpublished mouse studies (email Daniel for slides), SB002 was shown to be less cardiotoxic, has a high binding affinity to their putative target, reduce the m.13159T>G mutation in Parkinson’s fibroblasts, slow down the aging phenotype of POLG “mutator mice”, reduce blood glucose, heart weight, and the mean epigenetic age by 48% in some tissues.
The m.3243A>G mutation is also correlated with functional decline in aging, and risk of all-cause, dementia, and stroke mortality at heteroplasmy levels of 0 - 19% (Tranah et al. 2018).
Outlook for Shift Bioscience
Shift is currently developing a drug discovery platform that leverages single-cell CRISPR with single-cell aging clocks.
My Thoughts
Shift Bioscience is one of the more interesting mitochondrial longevity companies and it has a solid founder (Daniel Ives) with an incredible backstory, too. There are potentially a large number of rare mitochondrial diseases that could be addressed through Shift’s therapeutic approach.
And if mtDNA mutations truly drive aging then this technology would have enormous benefit. The single-cell CRISPR and single-cell epigenetic clock drug discovery platform Shift is developing also sounds fascinating -- would love to know more about it if possible.
GenSight Biologics (EPA:SIGHT)
Market cap: ~$327M USD
Enterprise Value: ~$281M USD
Cash: ~$46M USD
Founded: 2012
Modality: gene therapy, injection
Founders: Bernard Gilly, Botond Roska, Jose Alain Sahel, Luk Vandenberghe, Serge Picaud
Notable Investors: Abingworth, Index Ventures, Novartis Venture Fund, Versant Ventures
Pipeline:
Links
General Notes
GenSight Biologics is a Paris-based company that develops gene therapies to cure blindness.
The company’s lead program is LUMEVOQ (GS010), an AAV-based gene therapy to treat Leber’s Hereditary Optic Neuropathy (LHON). LHON is a rare disease of blindness caused by a mutation in the ND4, ND1, or ND6 mitochondrial genes that code for subunits of Complex I. LUMEVOQ expresses the wild-type ND4 mitochondrial gene in the nucleus.
GenSight’s LUMEVOQ is a proof of concept of allotopic expression of mitochondrial genes in the nucleus as a way to mitigate aging due to mitochondrial mutations.
GenSight also has future ambitions to use its proprietary mitochondrial targeting sequence to treat neurodegenerative diseases associated with mitochondrial dysfunction such as ALS or Parkinson’s.
GenSight is also in early-stage development of a treatment for dry age-related macular degeneration (Dry AMD) using an optogenetic approach -- not mitochondria-related but very cool nonetheless.
Origins and Funding
Based on the work of several researchers across many institutions developing gene therapy to treat various conditions of blindness. Founders include: Bernard Gilly, Botond Roska, Jose Alain Sahel, Luk Vandenberghe, Serge Picaud
$20 M Series A + $32M EUR Series B.
Pipeline Details
GS010 (LUMEVOQ) // Leber’s Hereditary Optic Neuropathy (LHON) // Phase 3
LHON is a rare mitochondrial disease that causes retinal ganglion degeneration, which leads to blindness. It is caused by a mutation in the ND4 (or ND1, ND6) mitochondrial gene that encodes for a protein in Complex I in the electron transport chain.
LHON affects roughly 1 in 50,000 people, mostly male (reason unknown). There are no FDA-approved treatments.
LUMEVOQ is an adeno-associated virus (AAV) vector gene therapy that encodes the cDNA of the mt-ND4 gene. Mitochondrial targeting sequences are used to encode short peptides (stolen from the COX10 mitochondrial protein gene) on the terminals of the ND4 gene that help guide the translated protein into the mitochondria (Cwerman-Thibalt et al. 2015).
GenSight has reported positive LUMEVOQ Phase 3 data, including a change in Best Corrected Visual Acuity (BCVA) of at least +15 letters compared to the lowest recorded BCVA in ~70% of patients (Newman et al. 2021). Interestingly, the treated and sham-treated eyes saw similar improvements. Given the size of improvement in BCVA vs the typical natural history of the disease, it is believed that the viral vector was able to migrate to the untreated eye.
GS010 has a good safety profile. No major adverse events. The main issues stem from the injection procedure (irritation, inflammation, etc).
GenSight filed for marketing approval with the EMA with a decision expected in Q4 2021. GenSight will submit a BLA with the FDA in H2 2021. Commercialization expected in 2022.
Expected cost for bilateral injection: 700,000 EUR. Market: ~1,500 new patients / year.
GS030 // Retina Pigmentosa, Dry age-related macular degeneration (Dry AMD), Phase 1/2
Both Retinitis Pigmentosa and dry AMD are diseases of blindness associated with progressive loss of photoreceptors.
Dry AMD is the leading cause of blindness in adults. It is characterized by degeneration of retinal pigment epithelial (RPE) cells and photoreceptors in the retina. Its exact cause is unknown but may be linked to dysfunctional mitochondria in RPE cells, which could lead to impaired nutrient and waste regulation (Kaarniranta et al. 2019).
Dry AMD affects ~300,000 new patients every year. Prevalence of 3.5 % in those aged 75+ and 22%+ in those 90+ years old.
GS030 is a gene therapy engineered to express a light-sensitive channelrhodopsin protein (from algae) in retinal ganglion cells, effectively turning the cells into rudimentary photoreceptors (Duebel et al. 2015). However, patients will require biomimetic goggles to amplify light and ensure proper light spectrum.
Positive Phase 1 safety data for GS030. No adverse events up to one year after gene therapy was administered.
Not really a therapy that targets mitochondria, even if the cause may be linked to mitochondrial dysfunction.
Other Future Applications
GS010 / mitochondrial targeting sequence tech: Parkinson’s disease, ALS, Leigh Syndrome
GS030: Vagus Nerve Stimulation, Congenital Deafness.
Outlook for GenSight Biologics
EMA approval decision for LUMEVOQ expected in Q4 2021. GenSight will submit a Biologics License Application with the FDA in H2 2021. Commercialization expected in 2022.
GS030 treatment for Retinitis Pigmentosa Phase 1/2 enrollment finished in 2020, preliminary data might be expected this year.
Future considerations: Using mitochondrial targeting sequences to treat neurodegenerative diseases associated with mitochondrial dysfunction such as Parkinson’s disease or Leigh’s syndrome.
My Thoughts
GenSight Biologics is one of the most interesting biotech companies I have stumbled upon. It doesn’t look like their roadmap plans for allotopic gene expression to solve aging caused by mitochondrial mutations but the demonstration in GS0101 for LHON was a great proof of concept that it can be done. GenSight’s GS030 treatment using channelrhodopsin and biomimetic goggles is really out-of-the-box thinking -- I love it.
Mitochondria and Metabolism
CohBar (NASDAQ:CWBR)
Market cap: $116M
Enterprise Value: $93M
Cash: $23M
Founded: 2007
Modality: peptides (from the mitochondrial genome), injection
Founders: Nir Barzilai, Pinchas Cohen
Notable Investors:
Pipeline:
Links
*I own a small amount of CohBar stock.
General Notes
CohBar is a company founded by Nir Barzilai and Pinchas Cohen that is developing novel mitochondrial-derived peptides (MDP) to treat diseases of aging and metabolic disease.
MDPs are short peptides that were recently discovered lurking in the mitochondrial genome (humanin in 2001, MOTS-c in 2015, and then many more). MDPs are believed to regulate mitochondrial function and metabolism.
CohBar has developed a platform for screening mitochondrial-derived peptides. Their compound library includes 100 novel peptides and 1000 analogs.
The company’s CB4211 peptide (an improved form of MOTS-c) is currently in Phase 1 trials to treat obesity and NASH (non-alcoholic steatohepatitis, i.e fatty liver disease). The peptide is believed to improve metabolism via sensitizing the insulin receptor. CohBar is also in the preclinical stage for peptide therapies for COVID ARDS, fibrotic disease, cancer, and type 2 diabetes.
Origins and Funding
The company was spun out of the research of Pinchas Cohen, a Professor at USC Leonard Davis School of Gerontology, and Nir Barzilai, Professor at Albert Einstein College of Medicine and father of the TAME trial. The two discovered the MOTS-c mitochondrial peptide (and many other peptides encoded in the mitochondrial genome) and its effects on insulin regulation and obesity.
CohBar raised $11M in an IPO in 2015. Also had a $15M dilutive stock offering in August 2020.
Pipeline Details
General mechanism of CB4211 (analog of MOTS-c)
MOTS-c is a 16-amino acid mitochondrial-derived peptide that regulates metabolism partly through the AMPK pathway and also directly by translocating to the nucleus and regulating nuclear gene expression (several antioxidant regulating genes, and ~800 other genes) as a response to metabolic stress. (Kim et al. 2018). AMPK is an enzyme that increases glucose and fatty acid uptake in response to low cellular energy.
Pinchas Cohen believes that MOTS-c can be thought of as an exercise mimetic. MOTS-c has been shown to reduce insulin insensitivity and obesity in mice that were fed a high-fat diet. It also increases glucose utilization and fatty acid oxidation and increases AMPK activation. It has no such effect on mice fed a normal diet, however. (Lee et al. 2015). Additionally, exercise increases endogenous MOTS-c levels in skeletal muscle and blood plasma and improves running capacity in young and old mice. Late-life intermittent treatment of MOTS-c also increases grip strength, gait, and 60-second walking tests of old mice. (Reynolds et al. 2021)
CB4211 // NASH(fatty liver disease) // Phase 1
NASH (non-alcoholic steatohepatitis) is liver inflammation and damage that arises from the accumulation of fat in the liver. It can lead to fibrosis, cirrhosis, liver cancer, and liver failure. It is a type of non-alcoholic fatty liver disease (NAFLD).
NASH is estimated to affect ~ 30% of type 2 diabetic patients and roughly 5% of the US total population.
The pathogenesis of NASH is not fully known but is linked to a myriad of different causes including mitochondrial dysfunction. (Caligiuri et al. 2016)
There are no FDA-approved drugs to treat NASH. 2020 saw a high profile FDA rejection of Intercept’s OCA treatment for NASH.
CohBar is currently conducting a Phase 1 clinical trial testing safety and pharmacodynamics/kinetics on non-obese patients that have NASH. The proof of concept trial will also measure the change in body weight, liver fat, and metabolic biomarkers (glucose, insulin, triglycerides, etc)
CB4211 (analog of MOTS-C) has shown to reduce fatty liver scores by 33% in STAM mouse models of NASH.
CB4211 // Obesity // Phase 1
Testing CB4211 on patients with obesity and non-alcoholic fatty liver disease ( >10% liver fat).
The obesity trial will be Part 3 of the CB4211 study of NASH (see above).
Other preclinical and discovery assets
CB5138 Antifibrotic Peptides (possible indications include IPF, idiopathic pulmonary fibrosis)
Positive results in vitro and in animal models of IPF -- reduced Ashcroft score for lung fibrosis and reduced biomarkers of fibrosis (alpha-smooth muscle actin, collagen I and II). (poster, 2020 ATS)
Current FDA-approved IPF treatments only slow the progression of the disease.
Apelin agonists (T2 Diabetes, ARDS Covid)
CXCR4 Inhibitors (Cancer)
Immunotherapy peptides for cancer
Outlook for CohBar
Data for Phase 1 trial of NASH and obesity expected in Q2 2021.
Finalizing their peptide candidate for anti-fibrotic disease, likely IPF indication.
My Thoughts
CohBar has an interesting platform (mitochondrial-derived peptides) with a good IP moat --nobody else is developing these peptides. The handful of MOTS-c mouse studies are promising but the fact that these peptides need to be injected could be an issue for widespread uptake. Dilutive stock offerings are also of concern from an investor standpoint. An interesting little note: The founders, Nir Barzilai and Pinchas, own 17% of the company.
Pano Therapeutics
Funding stage: Seed / Pre-seed
Total Funding: ?
Founded: 2017
Location: San Francisco, California, USA
Modality: small molecule drugs, natural compounds
Founders/Team: Benjamin Gibson, Francesca Fieni, Michael Zemel
Notable Investors: Longevitytech.fund
Pipeline:
Natural compound // Cardiometabolic indication // Pre-clinical
Synthetic compound // Acute therapeutics, cancer // Pre-clinical
General Notes
Pano Therapeutics is a pre-clinical stage company that is developing small molecule drugs / natural compounds to target a novel potassium ion channel in Complex I of the mitochondria. They believe their drug can mimic the mechanism of metformin, a diabetes drug that decreases blood glucose and extends lifespan in mice by ~6%.
Co-founders include Francesca Fieni (formerly at UCSF), Ben Gibson (Canaccord Genuity Group), and Michael Zemel (professor of Biochemistry at the University of Tennessee).
Investors include Longevitytech.fund, a Czech-based VC firm.
https://pubmed.ncbi.nlm.nih.gov/20551319/
Origins and Funding
Pano Therapeutics was founded in 2017 based on the research that co-founder Francesca Fieni conducted at UCSF regarding the discovery of a novel potassium ion channel associated with Complex I in the mitochondria.
The other two co-founders of Pano: Ben Gibson, CEO and co-founder, has an investment background as a director at Canaccord Genuity Group. Michael Zemel (co-founder) is a professor of Biochemistry and founder of NuSirt -- another longevity biotech company.
The company is backed by Longevitytech.fund, a Czech-based VC firm.
Pipeline Details
General: Targeting a mitochondrial ion channel, a better version of metformin.
Pano has yet to publish a paper on the specific compounds they are developing or the ion channel Fieni discovered but some details can be found in their patent filing and presentation at Metabesity 2020.
Pano wants to develop superior versions of metformin. Metformin is a diabetes drug that lowers blood glucose levels and potentially has an anti-cancer effect. It also extends the lifespan of mice by +6%. However, metformin has certain drawbacks (mostly active in liver tissue, bad cell membrane permeability, patent expiration hinders further clinical development).
Metformin’s mechanism is not fully understood. However, Pano believes they have discovered a novel mitochondrial ion channel in Complex I that is the fundamental target of metformin.
Pano has developed two programs to drug this novel mitochondrial ion channel:
A natural compound formulation to treat cardiometabolic disease
A synthetic drug to treat cancer
Natural compound // Cardiometabolic indication // Pre-clinical
Targeting novel ion channel associated with Complex I.
Combination of two known food compounds-- likely good safety profile. Planned for chronic applications.
Aiming to follow Amarin’s model of bringing a natural compound (omega-3) to market as pharmaceutical therapy. Pano intends to label expand to bigger markets with the eventual goal of an OTC / Off-label product.
Synthetic compound // Pancreatic and colorectal cancer (hypoxic tumours) // Pre-clinical
Optimized for acute administration
Plano claims they have positive in vitro studies in hand
Outlook for Pano Therapeutics
Pano is aiming to file an IND for their natural compound in Q2 2021. Indications include cardiometabolic conditions and cancer.
A forthcoming scientific publication of the discoveries that form the basis of their company (novel mitochondrial ion channel, lead molecules) has been promised.
My Thoughts
Longevity researchers and biohacking junkies alike have high hopes for metformin. But realistically speaking metformin’s lifespan extension effect on humans is likely to be very modest (it’s only +6% for middle-aged mice). A superior version of metformin sounds promising but we really need to see some in vivo data from Pano Therapeutics before making any judgment. A mouse lifespan study of their compounds would be tremendous.
Continuum Biosciences
Funding Stage: Seed
Total Funding: $500k
Founded: 2017
Location: Blacksburg, Virginia, USA
Modality: small molecule drugs, mitochondria uncoupler, oral
Founders/Team: Webster Santos, Kyle Hoehn
Notable Investors: Life Biosciences
Pipeline:
BAM15 // NASH, metabolic or age-related disease // Pre-clinical
General Notes
Continuum Biosciences is a preclinical-stage company based in Blacksburg, Virginia developing small molecule drugs that target mitochondria through depolarization/ uncoupling to treat metabolic and age-related diseases.
The company’s lead program is BAM15 (pre-clinical), an orally administered small molecule protonophore/mitochondria uncoupler that increases metabolism and reduces obesity in mice.
Continuum Biosciences is a daughter company of David Sinclair’s Life Biosciences. It was founded by Webster Santos (Professor of Chemistry at Virginia Tech) and Kyle Hoehn (Professor of Biochemistry at the University of New South Wales and University of Virginia)
Origins and Funding
Uncoupling proteins were first discovered in the early 1960s by studying brown adipose tissue (brown fat) in rodents. These first proteins were found to dissipate the mitochondrial membrane potential to divert the energy stored in glucose to produce heat instead of ATP. An uncoupler compound, 2,4 dinitrophenol (DNP), was once used as a weight-loss drug but was eventually prohibited due to its risk of death from toxicity and hyperthermia. Continuum is developing an improved version of DNP.
Continuum Biosciences was founded in 2017 by Webster Santos (Professor of Chemistry at Virginia Tech) and Kyle Hoehn (Professor of Biochemistry at University of New South Wales and University of Virginia). It is one of David Sinclair’s Life Bioscience’s daughter companies.
The company is based on Santos and Hoehn’s research on an improved small molecule oral protonophore/mitochondria uncoupler, BAM15, that does not have the unwanted side effects of DNP.
Pipeline Details
BAM15 // NASH, metabolic or age-related disease // Pre-clinical
Metabolic diseases (type 2 diabetes, obesity, fatty liver disease) are typically associated with insulin resistance, which has an increased risk with aging. Decreases in skeletal muscle, mitochondria dysfunction, oxidative stress, fat stores in muscle, inflammation, and decreased autophagy have all been linked to age-related insulin resistance.
Non-alcoholic steatohepatitis (NASH) is a fatty liver disease that affect ~5% of the US population. Metabolic syndrome is believed to affect 22% of the US population. Obesity affects 650M people worldwide.
BAM15 is a mitochondrial uncoupler drug -- it uncouples the mitochondria’s membrane potential from its ATP-generating function by transporting protons across the inner mitochondrial membrane back into the inner matrix. This decreases metabolic efficiency, which allows the mitochondria to “burn” more fuel without generating more ATP. Crucially, BAM15 only transports protons across the mitochondrial membrane, not the plasma membrane, unlike other decouplers (like DNP).
In a mouse model of diet-induced obesity, BAM15 was shown to be orally bioavailable, increase nutrient oxidation, decrease body fat mass without altering food intake or lean body mass, or body temperature. The mice lost 15% of their body mass, all of which came from fat. No toxicity was observed (Alexopoulos et al. 2020).
In another similar mouse study, BAM15 was shown to prevent diet-induced obesity and improve glycemic control independent of weight loss. Metabolic benefits were mediated by AMPK (Axelrod et al. 2020).
In a mouse model (STAM mouse) of non-alcoholic steatohepatitis (NASH), BAM15 was shown to decrease liver triglyceride levels and reduce fibrosis and inflammation. (Childress et at. 2020)
BAM15 has a 1.7h half-life, which will likely need to be improved.
Outlook for Continuum Biosciences
Unknown time frame for IND-enabling studies / clinical trials.
My Thoughts
Continuum has only one publicly-revealed program. However, a drug that acts through a fundamental mechanism to modulate metabolism has the promise of being a pipeline in a pill. Obesity, type 2 diabetes, and fatty liver disease (NASH) are large markets, some of which have limited therapeutic options. I’m curious how BAM15 will stack up against Novo Nordisk’s obesity drug, semaglutide.
Mitochondria Quality Control / Mitophagy / Biogenesis
Epirium Bio
Funding Stage: Series A
Total Funding: $85M
Founded: 2008
Location: San Diego, California, USA
Modality: small molecule drug, natural supplement mimetic, oral
Founders/Team: Pam Taub, Alan Maisel, Francisco Villarreal, Jonathan Taub, Sundeep Dugar, George Schriener.
Notable Investors: The Longevity Fund, ARCH Venture Partners, Adam Street Ventures, Longitude Ventures, Vertex Ventures, Bluebird Venture
Pipeline:
EPM-01 // Becker Muscular Dystrophy // Phase 1 (orphan drug status)
EPM-02 // Duchenne Muscular Dystrophy // Preclinical (orphan drug status)
General Notes
Epirium Bio is a San Diego-based clinical-stage company developing a synthetic flavanol, (+)-Epicatechin-- a molecule related to an antioxidant compound found in cocoa. Epicatechin is believed to stimulate mitochondrial biogenesis.
The company is initially targeting Becker Muscular Dystrophy in a Phase 1 clinical trial. Also has plans for a trial for Duchenne Muscular Dystrophy.
Many high-profile investors: ARCH Venture Partners, Longevity Fund, Vertex Ventures, Bluebird Ventures.
Origins and Funding
Formerly developing (-)-epicatechin to treat heart disease as Cardero Therapeutics. The company relaunched as Epirium Bio in 2019.
Epirium raised an $85M Series A in 2019. High-profile investors in the company include ARCH Venture Partners, Longevity Fund (Laura Deming), and Bluebird Ventures.
The company’s current pipeline is based on the research of George Schreiner and Sundeep Dugar on (+)-epicatechin and (-)-epicatechin, its relation to a novel human hormone associated with exercise, mitochondrial biogenesis, and muscle regeneration.
Epirium has conducted ~10 small pilot studies with epicatechins, which led them to initiate a clinical trial for Becker Muscular Dystrophy.
Pipeline Details
Mechanism of action: Catechins / Epicatechins.
Epirium is developing catechin drugs, compounds in the same family (isomers, epimers) as the flavonoid found in dark chocolate and green tea.
(-)-epicatechin is a natural flavonoid found in cacao, which has shown to counteract sarcopenia and stimulate mitochondrial biogenesis in mice. (+)-epicatechin, is a synthetic isomer of the (-)-epicatechin that Epirium believes to be more potent while having the same safety profile.
It is believed that (+)-epicatechin mimics 11-β-hydroxypregnenolone, a newly discovered natural hormone that interacts with cell surface receptors to stimulate mitochondrial biogenesis (Dugar et al. 2019).
(+)-Epicatechin (EPM-01) // Becker Muscular Dystrophy // Phase 1 (Orphan drug status)
Becker Muscular Dystrophy (BMD) is a rare genetic disease that causes general weakness that affects muscles in the hips, thighs, and shoulders. It is caused by mutations in the DMD gene that encodes dystrophin, a protein that connects muscle cells to the extracellular matrix. Loss of dystrophin is also linked to calcium overloading in mitochondria leading to cell death (Millay et al. 2008).
BMD is similar to Duchenne Muscular Dystrophy (DMD) but milder with a later onset. BMD affects roughly 18,000 people in the US and EU. There are no FDA-approved treatments for BMD or DMD.
In a preclinical study, mice fed (-)-epicatechin for 15 days saw ~50% improvement in treadmill performance, ~75% increase in mitochondrial volume density in muscle cells, and increased signaling factors of mitochondrial biogenesis (Nogueira et al. 2011).
A human pilot study of (-)-epicatechin treatment (100 mg/day for 8 weeks) demonstrated improvements in biomarkers associated with mitochondrial biogenesis and muscle regeneration (LKB1, AMPK, PGC1α, follistatin, etc) . However, there was no significant increase in 6-minute walking test results. The drug was well tolerated with headaches as the only related side effect. (McDonald et al. 2020).
Epirium initiated an open-label Phase 1 dose-escalation study of (+)-epicatechin for Becker Muscular Dystrophy in May 2020. The study is expected to finish in December 2021.
Other clinical studies:
Epirium is also planning clinical trials for (+)-epicatechin to treat Duchenne Muscular Dystrophy (DMD).
A small Phase 2 open-label study of (+)-epicatechin to treat Friedreich’s Ataxia showed non-statistically significant improvements in FARS/mFARS scores. However, there were improvements in cardiac function and upregulation of follistatin, a muscle-regeneration biomarker. (Quereshi et al. 2020)
Outlook for Epirium Bio
Phase 1safety trial of (+)-epicatechin to treat Becker Muscular Dystrophy to finish December 2021. Also planning a trial for Duchenne Muscular Dystrophy.
My Thoughts
Epirium is very interesting -- almost like a regenerative medicine approach using a natural-ish compound to increase mitochondrial biogenesis. The initial human data for (-)-epicatechin looks underwhelming but hopefully the (+)-epicatechin isomer will fare better.
Mitokinin
Funding Stage: ?
Total Funding: $5M
Founded: 2017
Location: New York, USA
Modality: small molecule drugs, PINK1 activator
Founders/Team: Nicholas Hertz, Daniel de Roulet
Notable Investors: Pfizer Ventures, Mission Bay Capital, Michael J. Fox Foundation, Abbvie (right to buy)
Pipeline:
MTK-458 // Parkinson’s disease // Pre-clinical (IND enabling)
MTK-458 // Huntington’s disease // Pre-clinical (in vivo)
MTK-458 // Alzheimer’s disease // Pre-clinical (in vitro)
General Notes
Mitokinin is a pre-clinical biotech startup that is developing small molecule drugs that increase the activity of the active-form of PINK1, a regulator of mitochondria quality control and mitophagy.
Mitokinin is targeting neurodegenerative diseases and is in IND-enabling studies for a therapy to treat Parkinson’s disease.
The company was co-founded by Nicholas Hertz, a former graduate researcher in Kevan Shokat’s lab at UCSF, whose research on PINK1 formed the scientific foundation of the company.
On March 2nd, 2021, Abbvie announced funding and an option to buy Mitokinin after the company completes its IND-enabling studies.
Origins and Funding
Founded in 2017 by Nicholas Hertz and Daniel de Roulet. Hertz was formerly a researcher in Kevan Shokat’s lab at UCSF. de Roulet is a serial life science and tech entrepreneur who previously co-founded Knowify.
Mitokinin is headquartered in New York City, USA. However, the company is a resident of UCSF’s QB3-MBC-BioLabs accelerator and a recipient of Amgen’s Golden Ticket, an award entailing lab access at MBC BioLabs.
The company has raised $5M including a $300k grant from the Michael J. Fox Foundation. Investors include Pfizer Ventures and Mission Bay Capital.
Pipeline Details
Method of Action: PINK1 activators help clear dysfunctional mitochondria
Mitokinin is developing a PTEN Induced Kinase 1 (PINK1) activator. PINK1 is a protein that is involved in maintaining mitochondria quality by removing dysfunctional mitochondria through mitophagy.
Healthy mitochondria have a membrane potential that pulls PINK1 into the inner membrane where it is degraded by the PARL protease. Unhealthy mitochondria have an insufficient membrane potential and are unable to draw in PINK1, letting it accumulate on the outer membrane of the mitochondria.
PINK1 proteins on the outer mitochondrial membrane recruit the Parkin protein, which signals autophagosomes to engulf damaged mitochondria and transport them to lysosomes for degradation. This process (mitophagy) clears “bad” mitochondria and their associated proteins thus regulating mitochondrial quality control.
In in vitro studies, Nicholas Hertz et al. discovered that kinetin (when converted into kinetin triphosphate in the cell) could increase the activity of PINK1/Parkin recruitment with even higher catalytic efficiency than ATP (the normal substrate it binds to) (Hertz et al. 2013). Kinetin was also shown to have the same effect on a known mutant version of PINK1 associated with Parkinson’s disease. Additionally, kinetin decreased mitochondrial motility, which is believed to be the first step in the process of removing damaged mitochondria (Wang et al. 2011). Lastly, kinetin was shown to be safe for dopaminergic neurons in vitro.
Mitokinin is now developing an improved version of kinetin to treat neurodegenerative disease. Kinetin itself is a plant-derived compound found in anti-wrinkle cream (likely safe).
MTK-458 // Parkinson’s disease // Pre-clinical (IND-enabling)
Parkinson’s disease is an age-related progressive neurodegenerative disease that leads to shaking, stiffness, and impaired walking, balance, and coordination. It is caused by the loss of dopaminergic neurons in the substantia nigra region of the brain.
Parkinson’s disease is diagnosed in 60,000 Americans each year and the lifetime risk is ~4%. Pharmacological treatments typically involve dopamine precursors or compounds that mimic dopamine.
Although the cause of Parkinson’s disease is not known, it has been linked to mitochondrial dysfunction. 90% of Parkinson’s disease cases do not have a known genetic basis, but the 10% that do can be traced to genes associated with mitochondrial dysfunction. (Reviewed in Park et al. 2018)
Kinetin, (MTK-458 analog), regulates mitochondria quality and was shown to reduce oxidative stress-induced cell death in dopaminergic neurons in mice (Hertz et al. 2013).
Other planned trials (neurodegenerative diseases)
MTK-458 // Huntington’s disease // Pre-clinical (in vivo)
MTK-458 // Alzheimer’s disease // Pre-clinical (in vitro)
Outlook for Mitokinin
Mitokinin had planned to submit an IND-filing with the FDA for MTK-458 to treat Parkinson’s disease in 2020, but this seems to have been delayed.
Mitokinin is also developing MTK-458 to treat Huntington’s disease and Alzheimer’s disease.
My Thoughts
Mitokinin’s approach of boosting the cell’s natural mitophagy process is clever. But the company hasn’t released any mouse model efficacy data as far as I know.
Other researchers have shown that kinetin does not protect against alpha-synuclein-induced neurodegeneration in PINK1 knockout mice (Parkinson’s disease model) (Orr et al. 2017). The authors of this study mentioned a number of caveats (the mouse model of Parkinson’s was not one of age-related neurodegeneration, the effect on mutant PINK1 gene was not tested) and reasoned the results of the in vitro studies in the original Hertz et al. paper still warranted human trials.
At any rate, I am curious to see what data they release. If the company is conducting IND-enabling studies they probably feel they have something -- Abvvie certainly sees something here. Kinetin, a natural plant compound, is believed to be fairly safe.
712 North
Funding Stage: Seed
Total Funding: $1M
Founded: 2016
Location: San Francisco, California, USA
Modality: small molecule drug
Founders/Team: Marcel Alavi
Notable Investors:
Pipeline:
328 // Glioblastoma // Pre-clinical
358 // Autosomal Dominant Optic Atrophy (AODA), Alzheimer’s // Pre-clinical
General Notes
712 North is an early-stage preclinical company developing personalized drugs that target mitochondria to treat cancer, Alzheimer’s disease, age-related diseases of the eye, and cardiovascular disease.
The company was founded by Marcel Alavi, a former postdoc at UCSF.
712 North is developing small molecules that can either inhibit or activate key enzymes (OPA1, OMA1) in mitochondrial homeostasis depending on the disease state of the patient.
712 North’s lead program is a small molecule for glioblastoma, an aggressive form of brain/spinal cord cancer. They are also developing a therapy to treat neurodegenerative and optic nerve atrophy diseases.
Origins and Funding
712 North was founded by Marcel Alavi and is based on his research at UCSF on mitochondrial homeostasis. In particular, Alavi discovered assays and biomarkers to measure mitochondrial homeostasis and inhibitors/activators of OMA1 OPA1 (key enzymes) to modulate mitochondrial homeostasis back to a normal state.
712 North is funded through a government SBIR grant from the NIH.
The company is presently a resident at the QB3 accelerator at UC Berkeley.
Pipeline Details
328 // Glioblastoma // Pre-clinical
Glioblastoma is an aggressive cancer that affects the brain or spinal cord. It has an incidence of ~1 in 30,000 people, most of which are 60+ years of age. The median survival time is ~15 months in patients who receive treatment. The market for glioblastoma is estimated to reach $1.4B by 2025.
328 is a modulator of OMA1, a protease enzyme that regulates the OPA1 protein through cleavage. OPA1 is a protein found in the inner mitochondrial membrane that plays a role in mitochondrial fission/fusion, quality control, and apoptosis.
Generally speaking, OMA1 activation promotes cell death and OMA1 inhibition protects a cell from death. But depending on a specific cancer disease state, the OMA1-OPA1 pathway may preferentially protect cancer cells over normal cells or vice-versa (Alavi 2019). By using either an OMA1 inhibitor for some patients and an OMA1 activator in others 712 North intends to treat cancer patients with a personalized therapy based on their disease state.
712 North has preclinical data (unpublished) showing reduced lymphoma cell-induced metastasis and increased mouse survival, reduced pancreatic bone metastasis, and inhibition of growth of patient-derived glioblastoma tumors in vitro. The company has also demonstrated that 328 can pass the blood-brain barrier. ~70% penetration rate.
358 // Autosomal Dominant Optic Atrophy (AODA), Alzheimer’s // Pre-clinical
Autosomal Dominant Optic Atrophy (AODA) is a rare disease that begins in childhood and mostly affects the optic nerve. It usually causes vision impairment but can also cause hearing loss, muscle weakness, and impaired coordination. It occurs in ~1 in 30,000 people. IAODA is caused by mutations in the OPA1 gene, which codes for a protein found in the inner mitochondrial membrane that a role in mitochondrial fission/fusion, quality control, and apoptosis
Alzheimer’s is a neurodegenerative disease that causes progressive impairment of memory and cognitive skills. It has a prevalence of ~10% in people 65+ years old.
The cause of Alzheimer’s is not known but the disease is associated with a build-up of protein aggregates in the brain (amyloid around brain cells, tau inside brain cells). Additionally, impaired mitochondrial fission and fusion balance and decreased levels of OPA1 are linked to Alzheimer’s diseases (Wang et al. 2009).
712 North plans to develop inhibitors/activators of OMA1 or OPA1 to regulate mitochondrial quality.
In some mouse models of neurodegeneration, the deletion of the OMA1 gene (remember OMA1 cleaves OPA1) delays neuroinflammation and neuron death and also prolongs lifespan independent of mitochondrial morphology (Korwitz et al. 2016).
Outlook for 712 North
Looking to raise an additional $4M Seed round to fund PK/PD and toxicology studies.
My Thoughts
712 North is still at a very early stage, working on a hypothesis involving OPA1-OMA1, mitochondria, and diseases such as cancer and optic nerve degeneration. The company has some mouse studies showing a reduction in cancer metastasis and increases in survival using 328 but they are unpublished. Need more information.
And….. I’ve broken Substack’s post length limit for real this time. Part 2 will follow immediately. Here, have a tweet: