#016: Every Single Longevity Therapy in Clinical Trial Today (PART 2).
OneSkin topical senolytic. Oxford Venture Capital Event. BONUS Database Access.
📡 In this edition of Longevity Marketcap Telemetry
Notable Last Week
Longevity Futures
Every Single Longevity Therapy in Clinical Trial Today (PART 2)
*Disclaimer: None of this should be taken as financial advice. This information is for educational purposes only.
*Warning: Investing in biotech is risky!
-Nathan Cheng @realNathanCheng
📝 Notable Last Week(s)
OneSkin releases study (pre-print) on their topical senolytic cream. The cosmetics industry has long been selling anti-aging products (of limited efficacy). But OneSkin brings something new to the table with a topical peptide cream they believe reduces senescent cell burden in the skin, according to their ex vivo data.
The paper needs to be peer reviewed, obviously. Also there was no randomized placebo clinical trial. But bringing anti-aging cosmetics developed in the context of longevity science is a pretty exciting development and I hope more companies start doing this — but with more definitive trials.
📅 Longevity Futures
November 16, 2020. 1:30 PM EST: Oxford Venture Capital Network Web Event: with Sebastian Brunemeier. Speaker is co-founder of Cambrian Biopharma, founder of Samsara Therapeutics, and previously investor at Apollo Ventures.
Every Single Longevity Therapy in Clinical Trials Today (PART 2).
Anti-aging therapies are already being tested in human patients today. What strategies are being tried? Which ones are likely to succeed?
Aging is a malleable biological process.
Scientists have been precisely turning the knobs of aging in model organisms since the 1990’s.
Presently we have reached an inflection point: Therapies developed in the context of longevity science are being tested in human patients today.
Continued from last time…
In my last post I reviewed the first 13 of the 28+ longevity therapeutics in clinical trials today, including pipelines from Unity Biotechnology, Alkahest, Samumed and more.
Let’s continue our analysis:
1. LYG-LIV0001 - LyGenesis
Status: Phase 2. Completion December 31, 2023.
Aging Target: Cell loss
Indication: End-stage liver disease
Endpoint: Restoration of liver function.
Primary / Secondary outcome: Engrafment efficacy. Liver function panel.
Modality: Ectopic liver regeneration.
Mechanism: Injection of allogeneic hepatocytes into patient lymph nodes to regenerate liver tissue.
Pre-clinical evidence:
Impact Factor: High.
Comments: Perhaps one of the more ambitious clinical trials. LyGenesis is a Juvenescence-backed startup spun out of research at the University of Pittsburgh. The company is attempting to regrow livers by injecting allogeneic liver cells into patient lymph nodes. The lymph nodes act as bioreactors and slowly regrow into functional livers — at least in the pre-clinical experiments done on pigs.
Their Phase 2 trial includes patients with end-stage liver disease. If successful the company plans to also use the same strategy to regrow the thymus (reversing immunosenescence) and also pancreatic islet cells (reversing diabetes).
2. BIO101 - BioPhytis (EPA:ALBPS)
Status: Phase 1. Study completion December 2020.
Aging Target: Altered intercellular communication.
Indication: Sarcopenia
Endpoint: Increased muscle function.
Primary / Secondary outcome measure: 400m walking test. Lean body mass. Hand grip test. Other physical performance tests.
Modality: Small molecule drug.
Mechanism: Activates MAS receptor. Stimulates(!) the P13K/AKT/mTOR pathway + AMPK/ACC pathway to grow muscle.
Pre-clinical evidence:
Impact Factor: Medium.
Comments: Biophytis is a French company that is attempting to reverse age-related loss of muscle mass. This is important not just for function in old age but also mortality: You are more likely to fall and break a hip when your muscles are deteriorated.
Their approach uses small molecule drugs that are similar to the chemicals plants produce in response to environmental stress like famines or disease. The hypothesis (David Sinclair coins “xenohormesis”) is that the signalling molecules in the stressed plants stimulate biological resilience in the animals that ingest them — an evolved survival response.
BIO101 is believed to target the Mas receptor in muscles to stimulate the P13K/AKT/mTOR pathway + AMPK/ACC pathway to grow muscle. This is a little paradoxical as many longevity drugs (like rapamycin) attempt to inhibit mTORC1. BIO101 also activates the AMPK pathway (regulates metabolism and autophagy), which is similar to how metformin is believed to extend lifespan.
3. Rapamycin - AgelessRx (PEARL Trial)
Status: Phase 4. Completion December, 2023.
Aging Target: Altered intercellular communication.
Indication: Aging.
Endpoint: Change in biomarkers.
Primary / Secondary outcome measure: Change in visceral fat. A number of blood biomarkers (CBC, liver panel, kidney panel, lipids, insulin, etc)
Modality: Small molecule drug.
Mechanism: Oral drug. Inhibit mTORC1, regulator of cell growth.
Pre-clinical evidence:
Impact Factor: Medium.
Comments: Rapamycin is one of the most studied life extending drugs. Initially used as an immunosuppressant for organ transplant recipients, it is known to reproducibly extend the lifespan of female mice by approximately 15%, even when administered in late life. (Rapamycin extends lifespan by 10% for male mice).
Unfortunately the patent on rapamycin expired in 1992 so there is little economic incentive to run clinical trials now. Various rapamycin analogs have been developed (resTORbio’s failed RTB101 is one of them) so there is still potential.
However one approach for a cost effective rapamycin trial is being spearheaded by AgelessRx, a direct to consumer longevity telehealth startup (think Hims but for longevity drugs). Their “PEARL” trial plans to test rapamycin treatment on 200 older patients for a total cost of $600,000 — partially funded through policy donors, crowd-funding, and $1000 participant fee.
The study will measure the changes in visceral fat, bone density, and various blood biomarkers (CBC, liver panel, kidney panel, blood glucose, insulin). And according to this Longevity.technology article they will also measure epigenetic methylation age, microbiome profile, and glycan age.
I think this is an interesting study that could pave the way for other low cost trials of other off-patent or natural longevity compounds. But the PEARL trial will not be able to measure lifespan extension in humans — only biomarkers of healthspan.
4. CD34+ cells enriched with MNV-BLD - Minovia Therapeutics
Status: Phase 1/2. Completion June 2021.
Aging Target: Mitochondrial dysfunction
Indication: Mitochondrial disease. Pearson Syndrome.
Endpoint: Reduction in Mitochondrial Disease scale.
Primary / Secondary outcome measure: Adverse events. Change IPMDS (International Pediatric Mitochondrial Disease Scale). Weight, height, organ function, etc.
Modality: Cell therapy. (autologous)
Mechanism: Extract CD34+ hematopoetic progenitor cells, infuse with healthy mitochondria from the mother, inject into patient — thus restoring balance of functional mitochondria vs mutated mitochondria.
Pre-clinical evidence:
Impact Factor: High.
Comments: Mitochondrial mutations are one of the 7 major SENS damage categories of aging identified by Aubrey de Grey. These mutations in the cellular “power plant” organelles accumulate with age and cause dysfunction and disease, in theory.
Minovia Therapeutics is attempting to solve the problem of mitochondrial mutations first with Mitochondrial Augmentation Therapy to treat Pearson Syndrome — an ultra rare and fatal mitochondrial disease. Minovia’s therapy involves extracting the blood forming stem cells from the patient, infusing them with healthy mitochondria from the mother, and injecting the cells back into the patient.
Minovia also has plans for an allogeneic version of this therapy (for inherited mtDNA mutations) which will lay down the foundations for tackling age-related mitochondrial diseases in the future.
5. rexlemestrocel-L, Revascor - Mesoblast (NASAQ:MESO)
Status: Phase 3. Completion March 1, 2021.
Aging Target: Degenerative disc disease.
Indication: Cell loss.
Endpoint: Reduction in low-back pain.
Primary / Secondary outcome measure:- Treatment Success (composite responder analysis of low back pain Visual Analogue Scale (VAS) score, Oswestry Disability Index (ODI) score and no post-treatment interventions)
Modality: Cell therapy. Stem cells.
Mechanism: Injection of allogeneic mesenchymal precursor cells into the vertebrae disc to reduce inflammation and stimulate regeneration.
Pre-clinical evidence:
Impact Factor: Medium.
Comments: Mesoblast is an Australian stem cell therapy company that focuses on allogeneic mesenchymal stem cells (and their lineages) to treat a number of different diseases. They achieve this mostly through the signalling factors that the stem cells release, which reduce inflammation and possibly induce regeneration.
Degenerative disc disease is a condition that is common and progresses with age. It is believed that one third or more of men and women between the ages of 40 - 59 have evidence of the condition. Not all people with signs of degenerative disc disease have pain or symptoms.
Stem cell therapy has already undergone a cycle of investor hype since the 1990s. But to date the FDA has not approved any stem cell therapies for non-cancer regenerative medicine. Hopefully, Mesoblast’s success can pave the way for future stem cell therapies.
6. SRK-015 - Scholar Rock (NASDAQ:SRRK)
Status: Phase 1 / 2. Completion April 2021.
Aging Target: Altered intercellular communication.
Indication: Spinal Muscular Atrophy.
Endpoint: Change in physical ability.
Primary / Secondary outcome measure: Change from Baseline in the Revised Hammersmith Scale. Change from Baseline in Hammersmith Functional Motor Scale Expanded (HFMSE)
Modality: Monoclonal antibody.
Mechanism: Inhibits the latent form of myostatin. Myostatin inhibits muscle growth.
Pre-clinical evidence:
Impact Factor: Medium.
Comments: Spinal Muscular Atrophy is a genetic disease that affects the survival of motor neurons. Muscles with decreased input from the central nervous system (CNS) slowly atrophy.
Scholar Rock is attempting to treat the disease by inhibiting the latent form of myostatin, a protein that inhibits muscle growth. The aim is to counteract the muscle atrophy from lack of CNS signalling with chemokine signalling that activates muscle growth.
There are already a number of FDA approved treatments for Spinal Muscular Atrophy (SMA), most targeting genetic expression / splicing directly. While Scholar Rocks therapy might not prove better than existing approaches for SMA, the potential of a general therapy that counteracts muscle atrophy has obvious potential benefits for aging.
7. SkQ1 - Mitotech
Status: Phase 3. October 2020.
Aging Target: Mitochondrial dysfunction
Indication: Dry eye
Endpoint: Improvement in eye comfort.
Primary / Secondary outcome measure: Ocular Discomfort Scale. Conjunctival Fluorescein Staining.
Modality: Small molecule drug.
Mechanism: SkQ1 is an antioxidant that penetrates the mitochondrial membrane to protect cardiolipin from reactive oxygen species.
Pre-clinical evidence:
Impact Factor: Medium.
Comments: Mitotech is an anti-aging company that develops therapies that protect the mitochondria from reactive oxygen species (ROS).
SkQ1 is an anti-oxidant that can easily penetrate the mitochondrial membrane where it can inhibit ROS. In particular, SkQ1 protects cardiolipin, an important protein found in the inner mitochondrial membrane.
Dry eye might not sound like a very sexy anti-aging therapy. But this trial is just one small step in the journey of treating mitochondrial diseases, many of which are age-related. Mitotech also has a pipeline for macular degeneration and NASH (non-alcoholic steatohepatitis) using a similar strategy.
8. Longeveron Mesenchymal Stem Cells (LMSCs) - Longeveron
Status: Phase 2. Completion July 31, 2021.
Aging Target: Cell loss. Stem cell exhaustion.
Indication: Frailty.
Endpoint: Improvement in ambulatory function.
Primary / Secondary outcome measure: Change from baseline in 6 Minute Walk Test (6MWT) compared to placebo
Modality: Cell therapy. Stem cells.
Mechanism: IV infusion of mesenchymal stem cells to reduce inflammation and promote regeneration.
Pre-clinical evidence:
Impact Factor: Medium.
Comments: Longeveron is a stem cell company that develops therapies for age-related disease. Frailty is an age-related condition that hinders physical function and also increases the risk of injury. It is usually measured in a clinical setting using an index that counts a person’s number of functional deficits from a standardized list. Typically frailty is highly correlated with mortality. Frailty is believed to affect 7 - 12% of people over the age of 65.
Longeveron is developing an allogeneic mesenchymal stem cell therapy infusion that they believe stimulates endogenous tissue repair and reduces inflammation. They also have a similar trial in Phase 1 to treat Alzheimer’s disease using the same therapy.
It seems that a lot of these mesenchymal stem cells therapy companies are light on mechanistic and specification details of their therapies. What differentiates one company’s allogeneic MSCs from another one? Perhaps they are trade secrets. I will need to investigate further.
9. Mesenchymal progenitor cells - Cellular Biomedicine Group (NASDAQ:CBMG)
Status: Phase 2. Completion January 2022.
Aging Target: Altered cellular communication. Cell loss.
Indication: Osteoarthritis (knee).
Endpoint: Reduction in self-reported pain.
Primary / Secondary outcome measure: WOMAC scores. MRI quantitative analysis of articular cartilage
Modality: Cell therapy. Stem cells.
Mechanism: Injection of human adipose-derived allogeneic mesenchymal progenitor stem cells to the knee. Stem cells stimulate self-repair and reduce inflammation.
Pre-clinical evidence:
Impact Factor: Medium.
Comments: Another allogeneic mesenchymal (progenitor) stem cell company — but this time with an operation focussed on the Chinese market. The company used to be a holding in Cathie Wood’s ARKG, but was fully dumped this year.
As with many of the other mesenchymal stem cell companies, they are very light on details of how the therapy works or what differentiates it from its competitors.
They use mesenchymal stem cells derived from human adipose (fat), which should be easier to harvest in theory.
10. Nicotinamide riboside - ChromaDex (NASDAQ:CDXC)
Status: Phase 1/2. Completion December, 2021
Aging Target: Genomic instability.
Indication: Inflammation. Acute illness.
Endpoint: Time to recovery.
Primary / Secondary outcome measure: Duration of stay of hospitalized patients. Normalization time for blood pressure and other vital signs.
Modality: Natural coenzyme precursor, small molecule. Oral.
Mechanism: Nicotinamide riboside is a precursor to NAD+. NAD+ is a crucial coenzyme required for many cellular processes, including the function of sirtuins that repair DNA strand breaks.
Pre-clinical evidence:
Impact Factor: Medium.
Comments: NAD+ boosting therapies are a hot topic and even more so ever since David Sinclair popularized it in his book and appearances on various podcasts. NAD+ is an essential coenzyme needed for many different cellular processes, including DNA repair via sirtuins.
NAD+ levels decrease with age so the hypothesis is that boosting NAD+ levels can have potential anti-aging benefits. There are several chemical precursors to NAD+ but the two most popular are nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN).
ChromaDex is the patent holder of NR and sponsors a number of clinical trials to support the case for its potential anti-aging properties. This time they are measuring hospitalization duration for patients with tissue damage. Given the small size of the study (84 participants) and probable heterogeneity in the illnesses, I have a hard time seeing how anything definitive will be elucidated.
It must be noted that NR seems to only increase healthspan in mice — not lifespan. For an exhaustive review of NAD+ boosting therapies, including all pre-clinical and human studies, see Forever Healthy’s review.
This is just one trial of MANY exploring the benefits of NAD+ precursors / booster. ChromaDex’s competitor, Elysium Health, is also running a number of trials with their NR + pterostilbene (anti-oxidant) supplement called “Basis”.
These NAD+ precursor compounds are generally considered safe so I’m excited to see what can benefits can be demonstrated in trials.
11. Mesenchymal stem cells, CRATUS - University of Miami
Status: Phase 1/2. Completion December, 2020.
Aging Target: Cell loss. Altered cellular communication.
Indication: Frailty
Endpoint: Safety. Decrease in frailty.
Primary / Secondary outcome measure: Adverse events, Difference in rate of decline of Frailty, Death from any cause, Exercise change in ejection fraction. Inflammatory Markers.
Modality: Cell therapy. Stem cells
Mechanism: IV infusion of allogeneic mesenchymal stem cells that reduce inflammation and stimulate endogenous tissue repair.
Pre-clinical evidence:
Impact Factor: Medium.
Comments: Another mesenchymal stem cell therapy trial! Very similar to the Longeveron trial for frailty, but this time conducted by Joshua Hare at the University of Miami.
Conclusions
I’m a liar. These two articles did not cover “every single longevity trial” currently planned or active.
I left out some trials conducted by the same company within the same pipeline or therapy.
I left out some trials because they lacked a lot of detail in the therapy.
I left out a large number of trials in the supplement / NAD+ space. These can mostly be grouped together.
I’m guessing I missed some mesenchymal stem cell therapy trials. Everyone is doing one.
Overall, I am both disappointed and encouraged by the number longevity clinical trials. From a big picture view it is incredible that we are actually testing longevity therapies in humans today.
But I will always be disappointed because we could always be doing more trials. The limiting factor is clearly not our imagination of what could be tested. The bottleneck is the cost of trials.
The logical conclusion is to create a better testing model that is more predictive than mice but less expensive than testing on humans. More on this later.
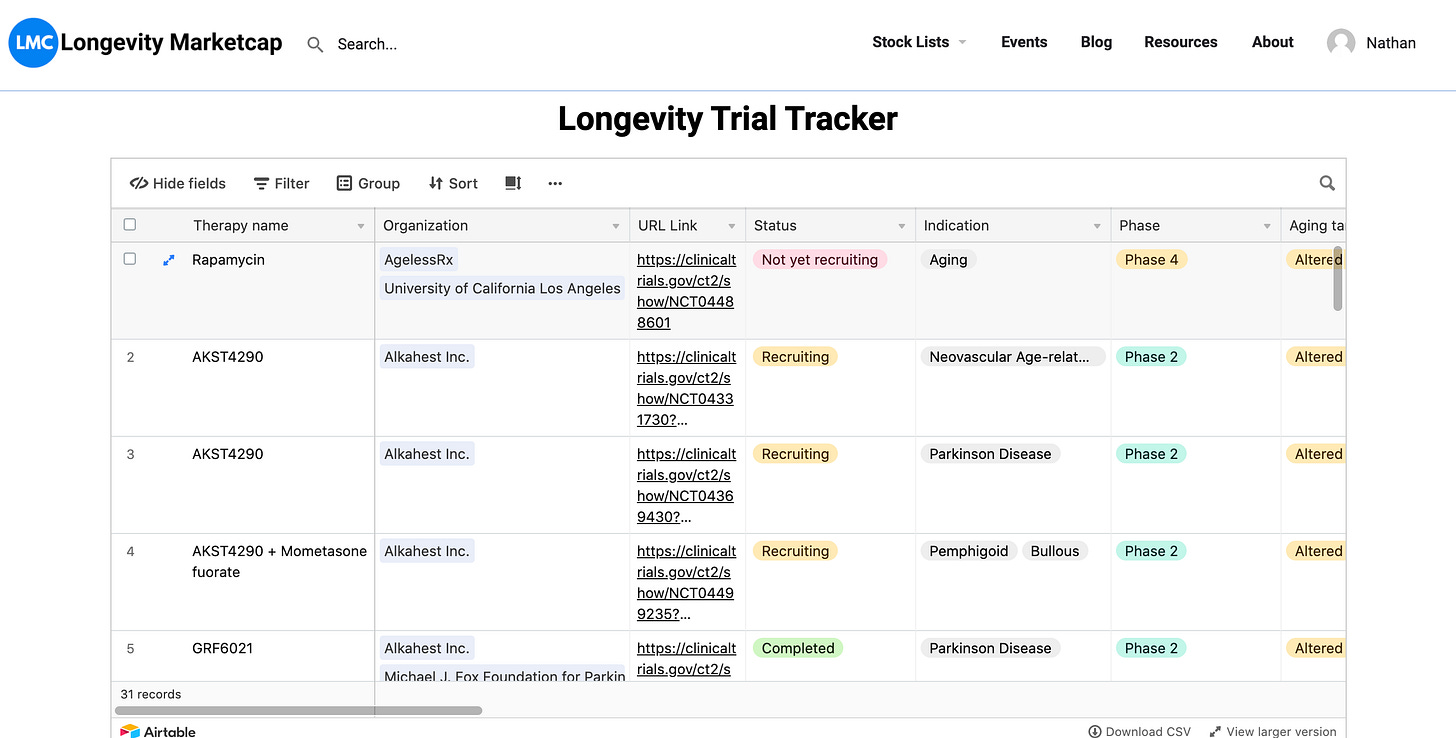
Bonus Access to Longevity Trial Airtable Database
I am going to give my readers access to my continually updated database of longevity trials, made with Airtable and a Python script that scrapes ClinicalTrials.gov. Just click the link.
The page also has a calendar view of the study completion dates. You can sync it to your own calendar app with this link.
-Nathan



Hey! You were featured on FightAging.org!!! That's quite the achievement IMO. Congrats
https://www.fightaging.org/archives/2020/12/a-tour-of-longevity-industry-therapies-presently-in-clinical-trials/
The Longevity Tracker spreadsheet is a great data source. Thanks!